1. Skootsky SA, Jaeger B, Oye RK. Prevalence of myofascial pain in general internal medicine practice. West J Med. 1989; 151:157–160. PMID:
2788962.
2. Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998; 75:1–17. PMID:
9539669.

3. Benjamin M. The fascia of the limbs and back: a review. J Anat. 2009; 214:1–18. PMID:
19166469.
4. Stecco A, Macchi V, Masiero S, Porzionato A, Tiengo C, Stecco C, et al. Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat. 2009; 31:35–42. PMID:
18663404.

5. Domingo T, Blasi J, Casals M, Mayoral V, Ortiz-Sagrista JC, Miguel-Perez M. Is interfascial block with ultrasound-guided puncture useful in treatment of myofascial pain of the trapezius muscle? Clin J Pain. 2011; 27:297–303. PMID:
21317780.

6. Hong CZ. Lidocaine injection versus dry needling to myofascial trigger point: the importance of the local twitch response. Am J Phys Med Rehabil. 1994; 73:256–263. PMID:
8043247.
7. Chu J, Yuen KF, Wang BH, Chan RC, Schwartz I, Neuhauser D. Electrical twitch-obtaining intramuscular stimulation in lower back pain: a pilot study. Am J Phys Med Rehabil. 2004; 83:104–111. PMID:
14758296.
8. McDonnell JG, O'Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: a prospective randomized controlled trial. Anesth Analg. 2007; 104:193–197. PMID:
17179269.

9. Hebbard P. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg. 2008; 106:674–675. PMID:
18227342.

10. Van Zundert J, de Louw AJ, Joosten EA, Kessels AG, Honig W, Dederen PJ, et al. Pulsed and continuous radiofrequency current adjacent to the cervical dorsal root ganglion of the rat induces late cellular activity in the dorsal horn. Anesthesiology. 2005; 102:125–131. PMID:
15618796.
11. Abejon D, Reig E. Is pulsed radiofrequency a neuromodulation technique? Neuromodulation. 2003; 6:1–3. PMID:
22150906.
12. Bevacqua B, Fattouh M. Pulsed radiofrequency for treatment of painful trigger points. Pain Pract. 2008; 8:149–150. PMID:
18366471.

13. Tamimi MA, McCeney MH, Krutsch J. A case series of pulsed radiofrequency treatment of myofascial trigger points and scar neuromas. Pain Med. 2009; 10:1140–1143. PMID:
19594852.
14. Ye L, Mei Q, Li M, Gu M, Ai Z, Tang K, et al. A comparative efficacy evaluation of ultrasound-guided pulsed radiofrequency treatment in the gastrocnemius in managing plantar heel pain: a randomized and controlled trial. Pain Med. 2015; 16:782–790. PMID:
25715902.

15. Simons DG. New views of myofascial trigger points: etiology and diagnosis. Arch Phys Med Rehabil. 2008; 89:157–159. PMID:
18164347.

16. Simons DG, Travell JG, Simons LS. Travell & Simons' myofascial pain and dysfunction: the trigger point manual. 2nd ed. Baltimore: Williams & Wilkins;1999.
17. Jaeger B. Myofascial trigger point pain. Alpha Omegan. 2013; 106:14–22. PMID:
24864393.
18. Shankar H, Reddy S. Two- and three-dimensional ultrasound imaging to facilitate detection and targeting of taut bands in myofascial pain syndrome. Pain Med. 2012; 13:971–975. PMID:
22681185.

19. Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001; 94:149–158. PMID:
11690728.

20. Ware JE Jr. SF-36 health survey update. Spine (Phila Pa 1976). 2000; 25:3130–3139. PMID:
11124729.

21. French JL, McCullough J, Bachra P, Bedforth NM. Transversus abdominis plane block for analgesia after caesarean section in a patient with an intracranial lesion. Int J Obstet Anesth. 2009; 18:52–54. PMID:
18996002.

22. Tran TM, Ivanusic JJ, Hebbard P, Barrington MJ. Determination of spread of injectate after ultrasound-guided transversus abdominis plane block: a cadaveric study. Br J Anaesth. 2009; 102:123–127. PMID:
19059922.

23. Snidvongs S, Mehta V. Pulsed radio frequency: a non-neurodestructive therapy in pain management. Curr Opin Support Palliat Care. 2010; 4:107–110. PMID:
20440207.

24. Erdine S, Bilir A, Cosman ER, Cosman ER. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009; 9:407–417. PMID:
19761513.

25. Moffett J, Fray LM, Kubat NJ. Activation of endogenous opioid gene expression in human keratinocytes and fibroblasts by pulsed radiofrequency energy fields. J Pain Res. 2012; 5:347–357. PMID:
23055776.

26. Vallejo R, Tilley DM, Williams J, Labak S, Aliaga L, Benyamin RM. Pulsed radiofrequency modulates pain regulatory gene expression along the nociceptive pathway. Pain Physician. 2013; 16:E601–E613. PMID:
24077210.
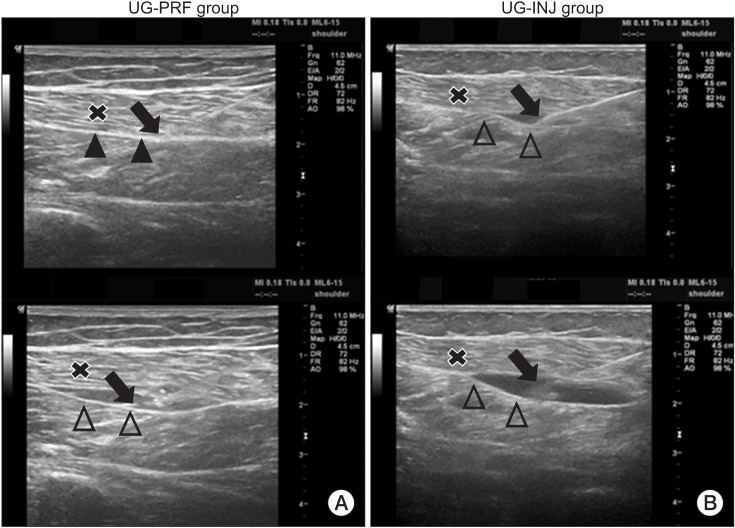
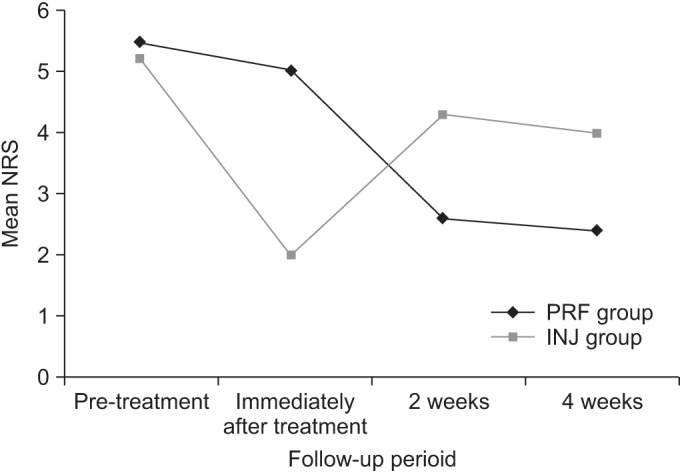
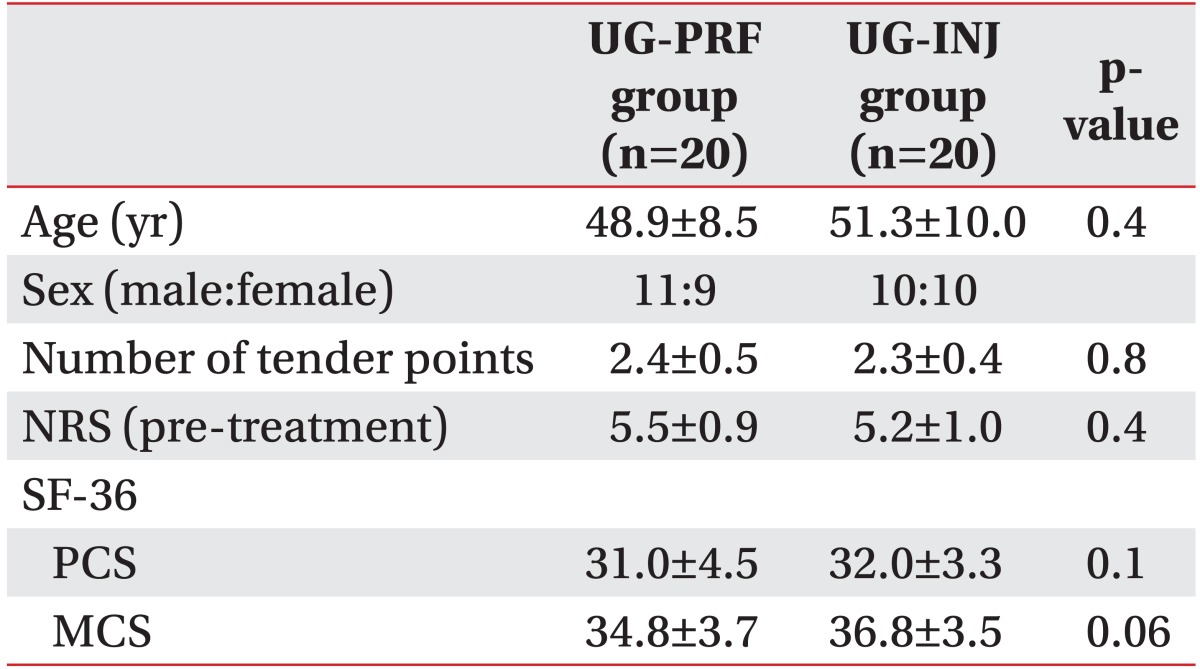
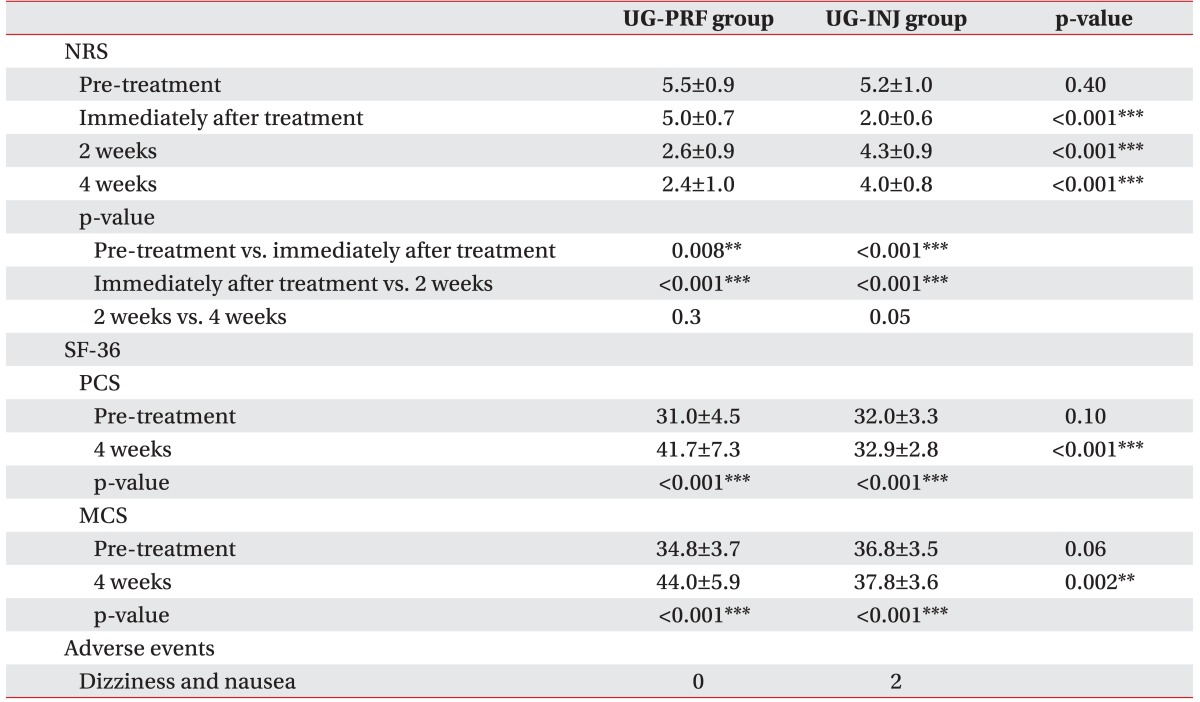




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download