Abstract
Objective
To develop a quantitative and organ-specific practical test for the diagnosis and treatment of dysphagia based on assessment of stroke patients.
Methods
An initial test composed of 24 items was designed to evaluate the function of the organs involved in swallowing. The grading system of the initial test was based on the analysis of 50 normal adults. The initial test was performed in 52 stroke patients with clinical symptoms of dysphagia. Aspiration was measured via a videofluoroscopic swallowing study (VFSS). The odds ratio was obtained to evaluate the correlation between each item in the initial test and the VFSS. A polychotomous linear logistic model was used to select the final test items.
Results
Eighteen of 24 initial items were selected as significant for the final tests. These 18 showed high initial validity and reliability. The Spearman correlation coefficient for the total score of the test and functional dysphagia scale was 0.96 (p<0.001), indicating a statistically significant positive correlation.
Dysphagia is closely associated with stroke, and aspiration pneumonia is a main cause of death in stroke patients [123]. Swallowing requires a harmonic movement of muscles in the mouth, pharynx and larynx in that order [4]. To evaluate the extent of dysphagia, it is necessary to evaluate cough reflexes, voice change after eating, speed and strength of mastication, sensation in the face and tongue, lip sealing, laryngeal elevation, and dysphonia [5]. Organ-specific treatment is very effective in treating dysphagia, according to Palmer et al. [6]. Various swallowing organs can be associated with aspiration, and thus, organ-specific assessment is helpful in establishing a treatment plan for stroke patients. Consequently, many dysphagia tests have been developed to assess the risk of aspiration pneumonia in stroke patients. Perry [7] suggested that the Burke dysphagia screening test, timed test, 3-oz water swallowing test, bedside swallowing assessment, and standardized swallowing assessment all provided useful screening tools. Recently, the Gugging Swallowing Screen (GUSS), Toronto Bedside Swallowing Screening Test (TOR-BSST), and acute stroke dysphagia screen have also emerged as useful screening tools [8910]. However, the above-listed screening tools are designed to mainly assess the extent of aspiration; therefore, it is difficult to obtain detailed information on the causes of dysphagia and possible treatments. A functional dysphagia scale (FDS) is herein developed based on a videofluoroscopic swallowing study (VFSS) to diagnose dysphagia and quantitatively express swallowing function [11]. As this method requires the technological capabilities to perform a VFSS and poses risks of radiation exposure, it has the disadvantage that the procedure cannot be performed at the patient's bedside immediately following a stroke [111213]. On the other hand, fiberoptic endoscopic evaluation of swallowing (FEES) can be done at the bedside without a risk of radiation exposure and is very useful in identifying the conditions of the pharynx and larynx; however, it is less capable of evaluating the oral period and esophageal phase and is not quantitative [14]. Nevertheless, VFSS and FEES are gold standards in the confirmation of dysphagia and aspiration is the most crucial factor in these tests.
Given the above, this study aims to develop a practical assessment that allows evaluation of swallowing function in stroke patients with a risk of dysphagia in a quantitative and an organic-specific manner and evaluate their applicability.
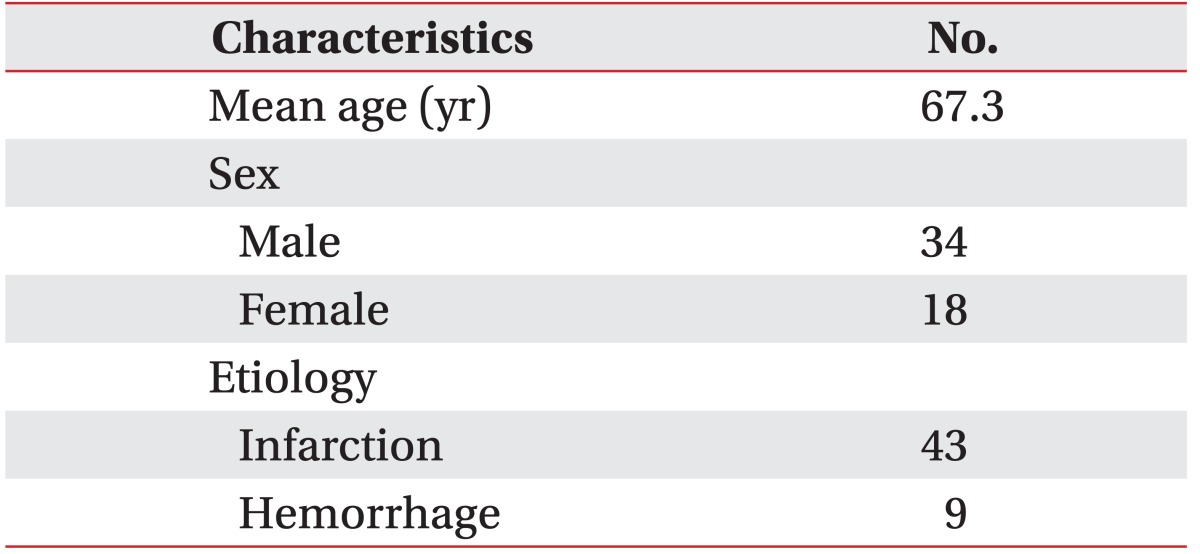
This is a prospective study that was approved by the Institutional Review Board of Chungbuk National University Hospital. A total of 52 stroke patients aged 20 or older who underwent VFSS to evaluate dysphagia in a rehabilitation center from May to June 2015 were involved in this study. The mean age was 67.3 years, and 34 male and 18 female patients participated in this study (Table 1). In stroke patients aged 20 or older who received a score of at least 14 on the Glasgow Coma Scale, the following cases were included in the study: 1) those with the symptoms of dysphagia including choking, coughing, and wet voice when eating, 2) those with a decreased gag reflex and laryngeal elevation or vocal cords paralysis, 3) those who underwent removal of a nasogastric tube preventatively after stroke onset, 4) those who would be considered useful in evaluating the recovery from dysphagia in stroke patients with nasogastric tubes. The following patients were excluded from this study: 1) patients with extremely low cognitive function, which would produce difficultly in maintaining a sedentary position for more than 10 minutes, 2) patients with a history of surgery on the oropharynx and esophagus, including tracheostomy, 3) patients suffering severe dysarthria or aphasia. A sample test was performed on 50 healthy adults without any medical history or symptoms of dysphagia in order to formulate the scoring system. The mean age was 63.6 years, and there were 24 males and 26 females.
This study was inspired by previous studies conducted by Mann et al. [15], therefore, it is composed of items designated by authors for the assessment of functions and organs thought to be highly associated with aspiration. The test is composed of items related to cognition, respiration, swallowing, and condition of the mouth, pharynx, and larynx. A single language therapist (Tester A) performed the test on the 50 normal patients. The means and standard deviations of each item were calculated. The scoring system for evaluation was thus designed. Then, the same language therapist (Tester A) performed the same test on stroke patients. About 31 patients were reevaluated by a rehabilitation physician (Tester B) to ensure reliability between the testers. Afterwards, another rehabilitation physician (Tester C) confirmed aspiration in patients via prospective VFSS. The testers were blinded to each other's results. The protocol developed by Han et al. [11] was used for the VFSS test and was the same for all patients.
The sample test was performed on 8 specific categories (cognition, respiration, lip, tongue, chin, soft palate, vocal cord, and swallowing). These 8 categories were further sub-divided into 24 items.
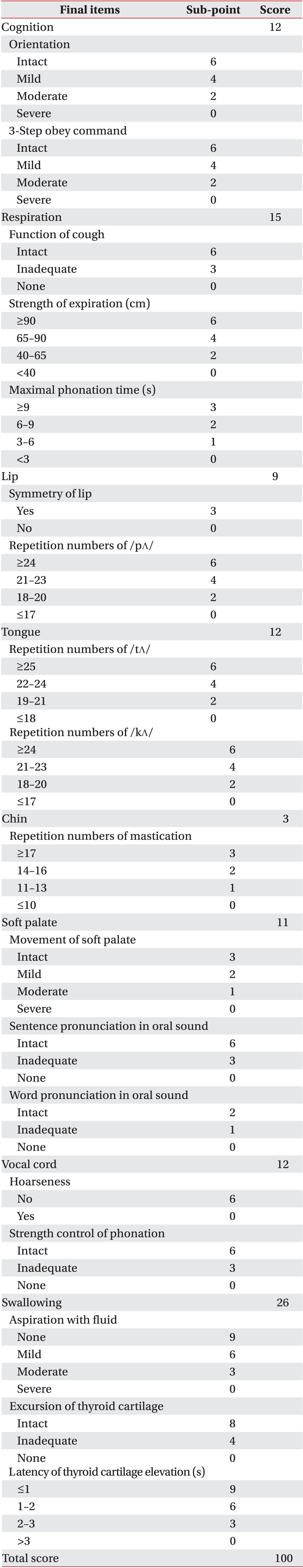
The cognition evaluation was composed of orientation analysis and the ability to obey a 3-step command. The patients were asked about time, place, and person to investigate their mental orientation and, based upon the accuracy of their answers, the severity of cognitive deficit was classified as follows: normal, all correct answers; mild, 2 correct answers; moderate, 1 correct answer; severe, all wrong answers. The 3-step command was used to evaluate whether the patients were able to follow 3 consecutive commands. The patient's ability to obey the commands was similarly ranked: normal, obeyed all commands; mild, obeyed 2 commands; moderate, obeyed 1 command; severe, failed to obey all commands.
Respiratory evaluation was composed of analysis of cough, maximum number of repeated expirations, strength of expiration, and maximum phonation duration. The patients were instructed to cough violently and the tester evaluated their coughing function on a 3-stage scale (normal, able to cough violently; moderate, able to cough but not perfectly; severe, unable to cough) based on his observations. In order to evaluate the ability for repeated expiration, patients were asked to repeatedly expirate as quickly as possible. Expiration was classified as follows: normal, at least 18; mild, 15 to 17; moderate, 12 to 14; severe, fewer than 11 expirations. The strength of expiration was assessed by the maximum distance at which a patient could blow out a candle while maintaining a seated position on a stationary chair. The strength of expiration was divided into 4 categories based on the distance: normal, at least 90 cm; mild, 65 to 90 cm; moderate, 40 to 65 cm; severe, less than 40 cm. The patients were asked to phonate as long as possible in a sedentary position to evaluate maximum phonation time. It was measured twice and the maximum of the two measurements was recorded. Phonation time was ranked as follows: normal, at least 9 seconds; mild, 6 to 9 seconds; moderate, 3 to 6 seconds; severe, less than 3 seconds.
The symmetry of the lips, ability to repeat /pΛ/, sensation in the lips, and drooling were evaluated to assess lip function. The symmetry of the lips was categorized as either normal (bilateral symmetry) or abnormal (bilateral asymmetry) based on the tester's observations. The patients were asked to pronounce the bilabial sound /pΛ/ as many times as possible in 5 seconds, and the number of pronunciations was recorded and ranked as follows: normal, at least 24; mild, 21 to 23; moderate, 18 to 20; severe, less than 17. The tip of a cotton bud was lightly applied to the patient's lips to evaluate sensation, which was classified in one of the following 3 categories : normal, same for left and right; moderate, different for left and right; severe, no sensation on either side. Drooling was classified as normal (no drooling) or abnormal (drooling).
The function of the tongue was evaluated for strength, number of times the patient could repeat the sounds /tΛ/ and /kΛ/, and sensation. A tongue depressor was used to examine the strength of the tongue as assessed by the patient's ability to raise the tongue depressor. Tongue strength was given one of 4 rankings: normal, able to withstand strong resistance; mild, able to withstand weak resistance; moderate, able to raise the tongue without resistance; severe, unable to raise the tongue at all. Ability to repeat /tΛ/ was evaluated by asking patients to pronounce the alveolar consonant /tΛ/ as many times as possible in 5 seconds. Based on repetition number, one of 4 rankings was given: normal, at least 24 repetitions; mild, 22 to 24; moderate, 19 to 21; severe, less than 18. Evaluation of the ability to pronounce /kΛ/ was measured in the same manner and again given one of 4 rankings: normal, at least 24; mild, 21 to 23; moderate, 18 to 20; severe, less than 17. To assess sensation, the tongue was touched lightly with the tip of a cotton ball. Sensation on the left and right sides were measured and one of 3 rankings was given: normal, same for left and right; moderate, different for left and right; severe, no sensation on either side.
The inter-incisor distance and mastication ability were measured in order to quantitatively evaluate the function of the chin. Patients were asked to open their mouths as widely as possible and inter-incisor distance was recorded. The distance was classified into 4 categories: normal, at least 3.5 cm; mild, 2.5 to 3.5 cm; moderate, 1.5 to 2.5 cm; severe, less than 1.5 cm. In order to examine the ability to masticate, the patients were asked to chatter their teeth as quickly as possible for 5 seconds, as they would if they were eating. Mastication was divided into 4 categories: normal, at least 17; mild, 14 to 16; moderate, 11 to 13; severe, less than 10.
The movement of the soft palate was observed, and the pronunciation of an oral sound was evaluated in order to examine the health of the soft palate. First, the patients were asked to pronounce /α/ while their tongue was pressed with a tongue depressor to evaluate the lifting and symmetry of the soft palate. The health of the soft palate was divided into 4 categories: normal, normal lifting and symmetry of the soft palate; mild, a slight decline in lifting of the soft palate or a slightly asymmetric soft palate; moderate, a significant decline in lifting of the soft palate or a severely asymmetrical soft palate; severe, no lifting of the soft palate at all. Second, the patients were asked to form words and sentences involving oral sounds, and their sound-making ability was divided into 3 categories: normal, accurate pronunciation of the oral sound; moderate, incomplete pronunciation of the oral sound with a slight nasal sound; severe, too much nasal sound, based on the tester's observations. The words used in this test were '학교, 파도, 가구, 버스, 카드, and 바다' in Korean. The sentence used was '바닷가에 파도가 거세게 쳐요' in Korean.
A tester evaluated the extent of hoarseness during conversation in order to evaluate the function of the vocal cords. Function was assessed as either normal (no hoarseness at all) or abnormal (hoarseness). The patients were then asked to increase the volume of their voices from a whisper to a yell in order to examine their ability to control strength of phonation. Control was given one of 3 rankings: normal, able to control phonation; moderate, difficulty in controlling phonation; severe, unable to alter the volume of their voice, based on the tester's observations.
A syringe was used to place distilled water on each patient's tongue, 3 mL each time 3 consecutive times. The patients were asked to swallow the water in order to evaluate the swallowing ability. Aspiration of fluid (choking) subjectively divided into 4 categories: normal, no choking or hoarse voice after swallowing; mild, no choking but slight hoarseness of voice; moderate, no choking but a clearly identifiable hoarseness of voice; severe, choking. Next, excursion of thyroid cartilage was given one of 3 rankings: normal, clear excursion of thyroid cartilage; moderate, slight excursion of thyroid cartilage; severe, barely any excursion of thyroid cartilage. Last, latency of thyroid cartilage elevation was measured as the mean value of the 3 repeats. The latency of thyroid cartilage elevation was given one of 4 rankings: normal, less than 1 second; mild, 1 to 2 seconds; moderate, 2 to 3 seconds; severe, 3 seconds or more.
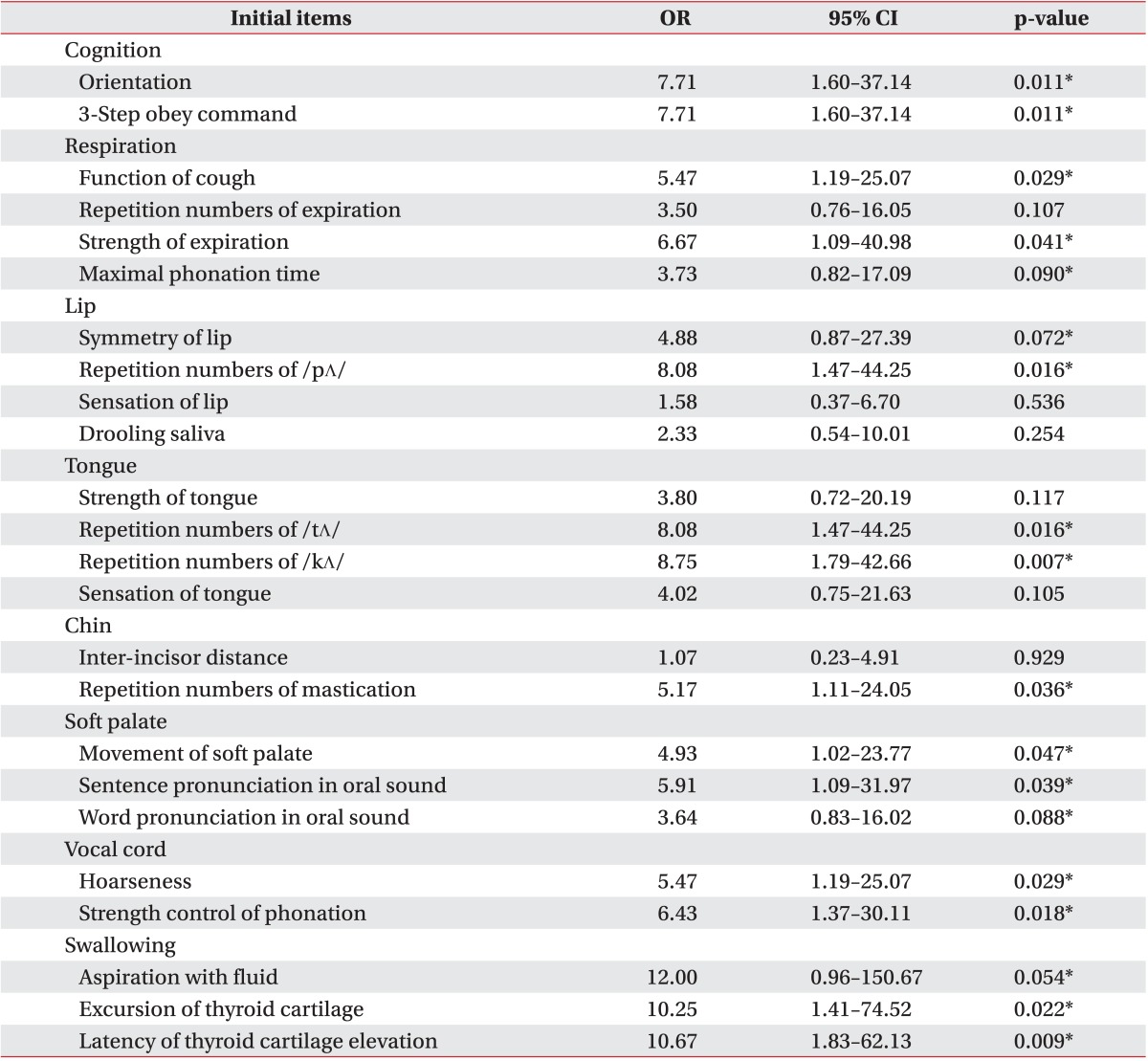
An overall evaluation was made by combining the means and standard deviations from each test item. The 'normal' state was based on the results of 50 healthy adults. The odds ratio between each evaluation item and the VFSS results was calculated to investigate the accuracy of the evaluation items. Each evaluation item was considered an independent variable, whereas aspiration on VFSS was the dependent variable. A polychotomous linear logistic model was used for regression analysis. Odd ratios, 95% confidence intervals (CIs) and p-values were obtained. From the initial 50 evaluation items, those with at least a 1.0 odds ratio and a p-value less than 0.1 were selected as final evaluation items. The final items were weighted on a relative scale. As a result, a 100-point evaluation was designed. Higher scores correspond with greater swallowing function. A receiver operator characteristic (ROC) curve was used to investigate the sensitivity, specificity, positive predictive value, negative predictive value and area under the ROC curve (AUC). The intra-class correlation coefficient (ICC) was used to evaluate inter-rater reliability, and a Spearman correlation coefficient was calculated to identify the relationship between total score and FDS score. The SAS ver. 9.3 program (SAS Institute Inc., Cary, NC, USA) was used to calculate all statistics.
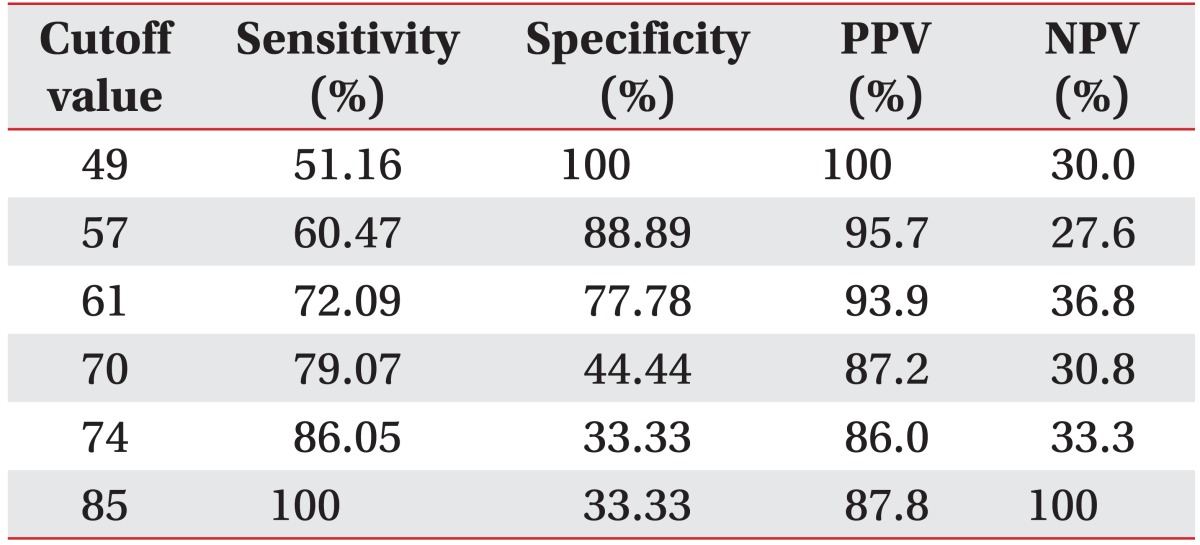
The most significant odds ratio observed was that between aspiration of fluid and the VFSS results (12.0) (Table 2). The odd ratios of all 24 evaluation items were 1.0 or above; however, the p-values for sensation of tongue, strength of tongue, repetition of expiration, drooling, sensation of lip, and inter-incisor distance were greater than 0.1, and therefore statistically insignificant. Thus, a total of 18 items with odds ratios greater than 1.0 and p-values less than 0.1 were selected as the final items in this test. Low scores indicate that the function of a particular organ declined, whereas higher scores refer to more satisfactory swallowing function, in general. Sub-points were given to sub-items (Table 3). Sensitivity of 51.16%, 72.09%, and 100%, specificity of 100%, 77.78%, and 33.33%, positive predictive values of 100%, 93.9%, and 87.8%, and negative predictive values of 30%, 36.8%, and 100% were observed at the cutoff points of 49, 61, and 85 points, respectively (Table 4). The AUC was 0.80, which indicates that the test was highly indicative of aspiration. In addition, the 95% CI was 0.671 to 0.902. The ICC between testers was 0.95, indicating high reliability (95% CI, 0.84 to 0.98). The Spearman correlation coefficient for the total score and number of FDS points of this test was 0.96 (p<0.001), which is a statistically significant positive correlation.
There is often difficulty ensuring that stroke patients receive sufficient nutrition because of a risk of aspiration pneumonia caused by dysphagia. Sufficient nutrition helps stroke patients recover, and early detection and identification of dysphagia is crucial for prognosis [1216171819]. As the importance of early detection of dysphagia has become ever more appreciated, various screens that can be performed at the patient's bedside have been developed [202122232425262728]. It has been reported that these screens are helpful in predicting potential aspiration pneumonia in mobility-impaired stroke patients [29]. The main purpose of such tests is to identify aspiration in the early stages of stroke; therefore, they are limited to the effort to initially evaluate dysphagia and set up a goal-oriented plan for later rehabilitation. Dysphagia is more accurately revealed when one takes into account the combination of several functional impairments than when one considers only a single impairment [30]. Therefore, it is absolutely necessary to develop a screen that helps to identify dysphagia and systematically and quantitatively evaluate the causes of dysphagia in stroke patients. Treatment becomes much more effective when applying a combined evaluation technique that identifies the causes of dysphagia in detail and helps to accelerate organ-specific rehabilitation.
A total of 24 evaluation items were identified that, in combination with one another, systematically and quantitatively evaluate the cognition, respiration, mouth function, pharynx health, larynx health, and swallowing ability in stroke patients. Then, a total of 18 items with odds ratios of at least 1.0 and p-values less than 0.1 were selected as the final items. As a result, a 100-point test was developed; higher scores on the test indicate more satisfactory swallowing function. Among the 18 items finally selected, a relatively high odds ratio was observed in the items related to the pharynx, such as aspiration of fluid, latency of thyroid cartilage elevation, and excursion of thyroid cartilage after swallowing distilled water. Therefore, we conclude that symptoms exhibited in the pharynx are of especial note in identifying dysphagia in stroke patients; this conclusion is in agreement with results from other studies done by Han et al. [11]. However, it should be noted that, as this study indirectly evaluates the latency of thyroid cartilage elevation and excursion of thyroid cartilage and we encountered difficulty in evaluating the placement of the tracheostomy tube accurately, patients with a tracheostomy tube were excluded.
We found a close relationship of cognition and 3-step command understanding with aspiration. Better cognitive function clearly contributes to these abilities, which are also considered to be associated with dysphagia. Patients were asked to pronounce words and sentences involving oral sounds and then evaluated for excess nasality in order to investigate the correlation of soft palate function with dysphagia. We assumed that the odds ratios of oral abilities in words and sentences would be similar. Unexpectedly, the odds ratio of the ability to pronounce words was lower than that of the ability to pronounce sentences. In healthy people, nasal output tends to reduce as the length of the stimulus word gets longer [31]. However, nasality in stroke patients clearly increased as the length of the stimulus word got longer in patients with incomplete closure of the soft palate, implying that the higher odds ratio for aspiration is related to the ability to pronounce oral phonemes in sentences.
The Burke dysphagia screening test, timed test, 3-oz water swallow test, bedside swallowing assessment, standardized swallowing assessment, GUSS, and TOR-BSST are the most commonly used screening tests today [78919212332333435]. The above tests include a common item, evaluation of coughing and wet voice in patients after feeding them water or test foods. Even so, the Burke dysphagia screening test, timed test, and standardized swallowing assessment are not in this sense in agreement with VFSS or FEES [21333435]. In addition, the sensitivities of the 3-oz water swallow test and bedside swallowing assessment were 76% and 68% with specificities of 59% and 67%, respectively. Thus, they are similar in sensitivity and specificity to this test at a cutoff point of 61 points [192332]. Those tests have not been evaluated for inter-tester reliability; therefore, the present test is considered to be more useful. GUSS and TOR-BSST are easily operable screening tests with higher sensitivities, validities, and reliabilities. However, the specificities of those tests were 50% and 66.7%, respectively, which are lower than those of this study [8936]. A precise assessment tool, VFSS-based FDS, developed by Han et al. [11] has 78.1% sensitivity and 77.9% specificity, similar to this study. However, our protocol has the extreme advantage of permitting evaluation without specialized VFSS equipment.
The Spearman correlation coefficient for total points and FDS score of the present test was 0.96 (p<0.001), which is a statistically significant positive correlation. FDS was developed using VFSS to confirm its validity [37], the present test is by extension also valid and useful. The AUC of this test was 0.80, which reveals relatively high validity and accuracy. The ICC for inter-rater reliability between tests was high (0.95), which indicates that it is a useful quantitative test with few errors caused by the subjective judgment of the tester.
The authors wished to use this study to identify, diagnose and set a treatment plan for dysphagia in stroke patients in an organ-specific manner; however, the authors have concluded that the this test is not suitable as a screening test since it has more evaluation items and requires far more hours to complete than other tests. It surpasses the usefulness limit of a screening tool [15]. Aspiration was only considered as a whole; therefore, a standardized score based on the amount and depth of each single aspiration, which may provide a faster means of evaluation, could not be assessed. The final test items were selected via odds ratios in comparison to aspirations on the VFSS test. Though aspiration was the most important variable in the swallowing process, it cannot represent all abnormalities in every organ. Development of a more complete assessment tool that reveals abnormalities in each organ is a worthwhile process that this study hopes to foster. If dysphagia is caused by a single organ while the remaining organs are normal, the total score of this test might be higher than the swallowing function in an actual patient, so special attention is required when analyzing and applying the results. This test is not able to evaluate the food residues and is limited in definition of delayed aspiration, which is must be improved in the future.
The significance of this study is that is systematically evaluated the contribution of each organ to dysphagia in stroke patients. Evaluating swallowing function in a quantitative and organ-specific manner is a highly useful tool in planning the treatment and observing the progress of stroke patients.
ACKNOWLEDGMENTS
This work was supported by the research grant of Chungbuk National University in 2014.
References
1. Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001; 16:7–18. PMID: 11213249.

2. Kidd D, Lawson J, Nesbitt R, MacMahon J. The natural history and clinical consequences of aspiration in acute stroke. QJM. 1995; 88:409–413. PMID: 7648232.
3. Hinchey JA, Shephard T, Furie K, Smith D, Wang D, Tonn S. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005; 36:1972–1976. PMID: 16109909.

4. Cook IJ, Dodds WJ, Dantas RO, Kern MK, Massey BT, Shaker R, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989; 4:8–15. PMID: 2640180.

5. Beom J, Han TR. Treatment of dysphagia in patients with brain disorders. J Korean Med Assoc. 2013; 56:7–15.

6. Palmer JB, Drennan JC, Baba M. Evaluation and treatment of swallowing impairments. Am Fam Physician. 2000; 61:2453–2462. PMID: 10794585.
7. Perry L. Screening swallowing function of patients with acute stroke. Part one: Identification, implementation and initial evaluation of a screening tool for use by nurses. J Clin Nurs. 2001; 10:463–473. PMID: 11822494.

8. Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke. 2007; 38:2948–2952. PMID: 17885261.
9. Martino R, Silver F, Teasell R, Bayley M, Nicholson G, Streiner DL, et al. The Toronto Bedside Swallowing Screening Test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009; 40:555–561. PMID: 19074483.
10. Edmiaston J, Connor LT, Loehr L, Nassief A. Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010; 19:357–364. PMID: 19875722.

11. Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001; 82:677–682. PMID: 11346847.

12. Song WW, Yi SH, Kim EJ, Kim HN, Park JJ, Choi KI, et al. Validation of gugging swallowing screen for patients with stroke based on videofluoroscopic swallowing study. J Korean Acad Rehabil Med. 2009; 33:704–710.
13. Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res. 1995; 38:556–563. PMID: 7674647.

14. Kim IS, Han TR. Evaluation and management of dysphagia. Korean J Stroke. 2006; 8:40–48.
15. Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000; 10:380–386. PMID: 10971024.

16. Kim S, Byeon Y. Comparison of nutritional status indicators according to feeding methods in patients with acute stroke. Nutr Neurosci. 2014; 17:138–144. PMID: 23863463.

17. Holas MA, DePippo KL, Reding MJ. Aspiration and relative risk of medical complications following stroke. Arch Neurol. 1994; 51:1051–1053. PMID: 7945003.

18. Horner J, Buoyer FG, Alberts MJ, Helms MJ. Dysphagia following brain-stem stroke. Clinical correlates and outcome. Arch Neurol. 1991; 48:1170–1173. PMID: 1953404.
19. Horner J, Massey EW. Silent aspiration following stroke. Neurology. 1988; 38:317–319. PMID: 3340301.

20. DePippo KL, Holas MA, Reding MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Arch Neurol. 1992; 49:1259–1261. PMID: 1449405.

21. Gottlieb D, Kipnis M, Sister E, Vardi Y, Brill S. Validation of the 50 ml3 drinking test for evaluation of poststroke dysphagia. Disabil Rehabil. 1996; 18:529–532. PMID: 8902426.
22. DePippo KL, Holas MA, Reding MJ. The Burke dysphagia screening test: validation of its use in patients with stroke. Arch Phys Med Rehabil. 1994; 75:1284–1286. PMID: 7993165.

23. Mari F, Matei M, Ceravolo MG, Pisani A, Montesi A, Provinciali L. Predictive value of clinical indices in detecting aspiration in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 1997; 63:456–460. PMID: 9343123.

24. Smithard DG, O'Neill PA, Park C, England R, Renwick DS, Wyatt R, et al. Can bedside assessment reliably exclude aspiration following acute stroke? Age Ageing. 1998; 27:99–106. PMID: 16296668.

25. Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Arch Phys Med Rehabil. 1999; 80:150–154. PMID: 10025488.

26. Sellars C, Dunnet C, Carter R. A preliminary comparison of videofluoroscopy of swallow and pulse oximetry in the identification of aspiration in dysphagic patients. Dysphagia. 1998; 13:82–86. PMID: 9513301.

27. Teramoto S, Fukuchi Y. Detection of aspiration and swallowing disorder in older stroke patients: simple swallowing provocation test versus water swallowing test. Arch Phys Med Rehabil. 2000; 81:1517–1519. PMID: 11083358.

28. Teramoto S, Matsuse T, Fukuchi Y, Ouchi Y. Simple two-step swallowing provocation test for elderly patients with aspiration pneumonia. Lancet. 1999; 353:1243. PMID: 10217091.

29. Smithard DG, O'Neill PA, Parks C, Morris J, Wyatt R, England R, et al. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. 1996; 27:1200–1204. PMID: 8685928.
30. Veis SL, Logemann JA. Swallowing disorders in persons with cerebrovascular accident. Arch Phys Med Rehabil. 1985; 66:372–375. PMID: 4004534.
31. Kim M, Sim HS, Choi HS. The effects of phonetic context and stimulus length on the nasalance score in normal adults. Korean J Commun Disord. 2001; 5:1–15.
32. Smithard DG, O'Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997; 12:188–193. PMID: 9294937.

33. Hinds NP, Wiles CM. Assessment of swallowing and referral to speech and language therapists in acute stroke. QJM. 1998; 91:829–835. PMID: 10024948.

34. Ellul J, Barer D. Interobserver reliability of a Standardised Swallowing Assessment (SSA). Cerebrovasc Dis. 1996; 6:152–153.
35. Perry L. Screening swallowing function of patients with acute stroke. Part two: Detailed evaluation of the tool used by nurses. J Clin Nurs. 2001; 10:474–481. PMID: 11822495.

36. Chun SW, Lee SA, Jung IY, Beom J, Han TR, Oh BM. Inter-rater Agreement for the Clinical Dysphagia Scale. Ann Rehabil Med. 2011; 35:470–476. PMID: 22506161.

37. Paik NJ, Kim IS, Kim JH, Oh BM, Han TR. Clinical validity of the functional dysphagia scale based on videofluoroscopic swallowing study. J Korean Acad Rehabil Med. 2005; 29:43–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download