Abstract
Methods
A total of 79 patients diagnosed with XGC were included in the study. The criteria for XGC in the pathology specimens were the presence of histiocytes, cholesterol deposits, lipids, and focal or widespread wall enlargement.
Results
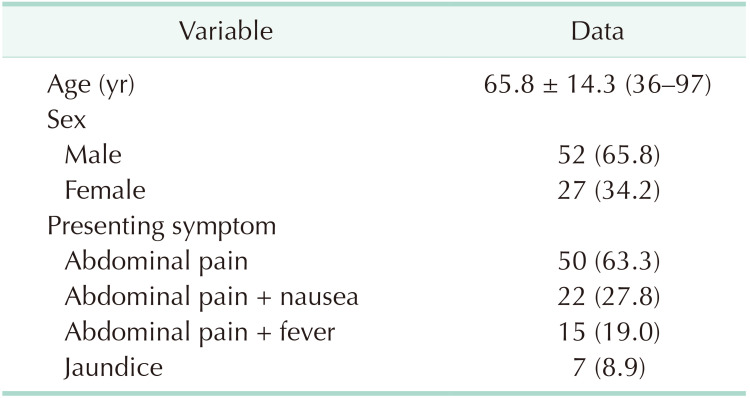
Patients were diagnosed with XGC, of which 52 (65.8%) were male and 27 (34.2%) were female, creating a male-to-female ratio of 2:1. The mean age was 65.8 ± 14.3 years (range, 36–97 years). The most common presenting symptom was abdominal pain (63.3%), and the least common presenting symptom was jaundice (8.9%). Of the total, 25 patients were found to have pathological conditions with the potential to obstruct the bile duct or to slow bile flow. A frozen section examination was performed on 20 patients due to suspicion of a tumor by intraoperative macroscopic examination. However, no malignancy was detected in the cases who underwent a frozen section examination. An increase in wall thickness of the gallbladder was observed in 81.6% (n = 31) of the patients on computed tomography scans and in 81.8% (n = 18) of the patients on magnetic resonance imaging scans in which possible tumor lesions were reported, but no tumor was detected.
Xanthogranulomatous cholecystitis (XGC) was first described as benign and pseudotumor of the gallbladder in 1970 by Christensen and Ishak [1]. It was in 1981 when it was first described as a distinct pathological condition by Goodman and Ishak [2]. Xanthogranulomatosis is an idiopathic, rare process in which lipid-laden histiocytes are deposited at various locations in the body. Xanthogranulomatous inflammation occurs in various organs such as skin, kidney, retroperitoneum, intracranium, gastrointestinal tract, genital organs, and gallbladder [34]. XGC is an unusual form of chronic cholecystitis that may simulate malignancy radiologically and pathologically [5]. Many studies have been performed investigating whether there are imaging findings that might permit differentiation of XGC from cancer, such as diffuse gallbladder wall thickening, intramural “nodules” related to macrophage deposition, an intact gallbladder mucosa, and calculi [56].
The clinical picture can take the form of acute or chronic cholecystitis and may manifest with different symptoms due to inflammation, fistula, and fibrosis. XGC may show similar findings to acute cholecystitis, chronic cholecystitis, or gallbladder cancer in such imaging techniques as ultrasonography (US), CT and MRI performed in the preoperative period [378]. Preoperative diagnosis of XGC is often difficult, and an intraoperative frozen section examination may be required in selected cases to distinguish it from malignancy. The clinical presentation resembles acute or chronic cholecystitis, and encountering difficulties in cholecystectomy is not uncommon [47]. In such cases, an open approach is frequently adopted due to suspicion of cancer or anticipation of significant difficulty and higher conversion to open cholecystectomy rate [37].
This study presents our clinical experience of patients with XGC, together with a review of literature.
The hospital files of 8,530 patients who underwent a cholecystectomy in our clinic between January 2003 and January 2020 were reviewed, and 79 patients diagnosed with XGC were included in the study. Patient files, hospital information system records, operation reports, pathology reports, and anesthesiology reports were reviewed, and the information was uploaded into a data system created for the purpose of this study. The cases were analyzed retrospectively, and follow-up data was supported by phone calls to the patients. This study was approved by the Institutional Review Board of Faculty of Medicine, Cukurova University (10.01.2020/95/22), and written informed permission was taken from every patient who registered in the study.
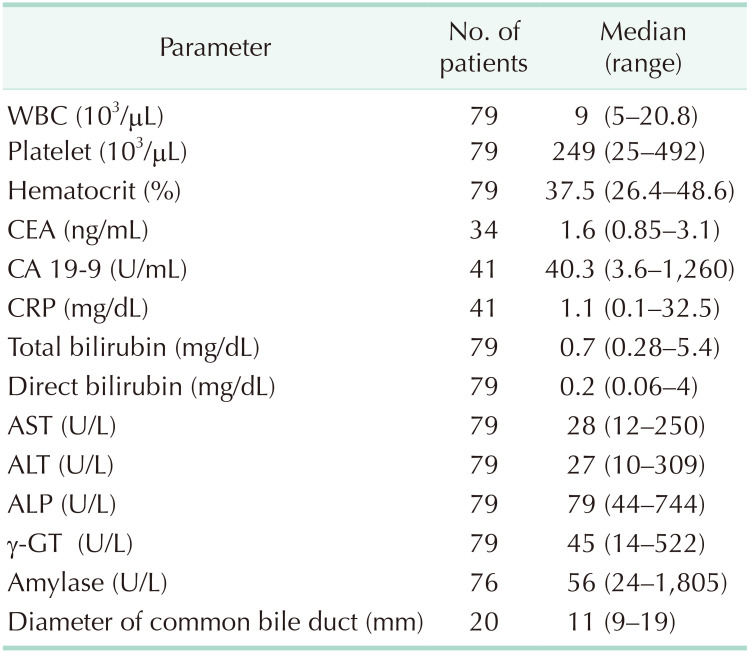
The demographic characteristics of the patients, comorbidities, presenting symptoms, preoperative laboratory values, tumor markers, preoperative radiological imaging findings, surgical procedures performed, intraoperative complications, operation time, rate of conversion to open cholecystectomy, postoperative complications, pathology reports, postoperative length of hospital stay, reoperation, postoperative 90-day mortality, postoperative 90-day unscheduled readmission to the hospital, mean duration of follow-up, survival, and current clinical condition of the patients were analyzed. Open cholecystectomy method was preferred in patients with suspected malignancy in the preoperative period and in patients with previous surgical history. Open cholecystectomy was performed because laparoscopic cholecystectomy (LC) did not become the standard treatment method in the first 5 years of this study in our clinic. The term “conversion to open cholecystectomy” refers to the operation starting with the laparoscopic method and then returning to open cholecystectomy. The common bile duct was considered to be dilated if the diameter was ascertained to be >7 mm in radiological imaging methods.
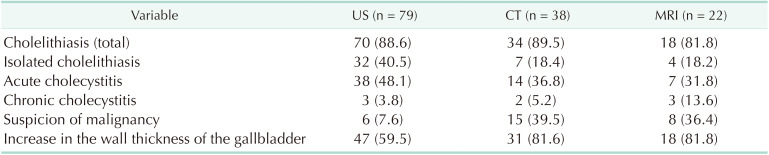
The criteria for XGC in the pathology specimens were the presence of histiocytes, cholesterol deposits, lipids, and focal or widespread wall enlargement; foreign body giant cells or Touton-type multinucleated giant cells; cells phagocytizing lipids and bile pigments to form xanthomatous cells; and the presence of acute and chronic inflammatory cells. The characteristic feature of XGC on gross examination of the surgical specimen was the presence of a lot of grayish-yellow nodules in the gallbladder wall upon opening of the gallbladder. Patients with inaccessible data, patients in whom a diagnosis of XGC could not be confirmed by a pathological examination, and patients aged <18 years were excluded from the study. Representative US image, CT image, and MRI image (Fig. 1), surgical specimenphotography (Fig. 2), and pathological micrographs of XGC (Fig. 3) are shown. Case-based evaluations based on anamnesis, physical examination, and laboratory findings were effective in the lesions with high index of suspicion for malignancy in the radiological imaging selection. The term “isolated cholelithiasis” was used only for patients with gallstones in the radiological imaging finding and without any other pathological findings in the US, MRI, and CT. The expression of increased wall thickness of gallbladder was used for all patients with acute cholecystitis, chronic cholecystitis, and suspicion of malignancy in the US, CT, and MRI.
The IBM SPSS Statistics ver. 23.0 software package (IBM Corp., New York, NY, USA) was used for the statistical analysis of the data. Categorical measurements were summarized as number and percentage, and continuous measurements were expressed as mean and standard deviation (with median and range where required). A Pearson chi-square test was used for the comparison of categorical variables. The level of statistical significance was set at an alpha of 0.05 for all tests.
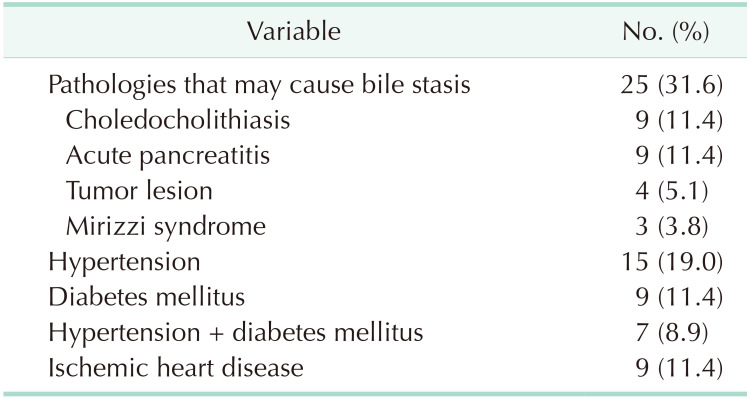
After the examination of cholecystectomy specimens, 79 patients (0.93%) were diagnosed with XGC, of which 52 (65.8%) were male and 27 (34.2%) were female, meaning a male-to-female ratio of 2:1. The demographic details of the patients and the presenting symptoms are shown in Table 1. The mean age of the patients was 65.8 ± 14.3 years (range, 36–97 years). The most common presenting symptom was abdominal pain (n = 50, 63.3%), and the least common presenting symptom was jaundice (n = 7, 8.9%). Some cases presented with more than 2 symptoms. The preoperative laboratory findings of the patients are presented in Table 2. There was no statistically significant difference in terms of laboratory findings. The median CA 19-9 level was 40.3 U/mL (range, 3.6–1,260 U/mL), and the median CEA level was 1.6 ng/mL (range, 0.85–3.1 ng/mL). The radiological imaging findings of US, CT, and MRI are given in Table 3. Cholelithiasis was detected in 70 patients (88.6%) on US, 34 (89.5%) on CT, and 18 (81.8%) on MRI. Malignancy was suspected in 6 patients (7.6%) on US, 15 (39.5%) on CT, and 8 (36.4%) on MRI. Of the total, 5 patients underwent 18F-fluorodeoxyglucose PET/CT with suspected tumor, and the mean maximum standardized uptake value was found to be 10 (range, 5.5–23.5). Of these patients, 3 patients underwent a frozen section examination during surgery, which was considered in favor of XGC, while a frozen section examination was not performed in 2 patients as malignancy was not considered during surgery. The comorbidities of the patients and the accompanying hepato-pancreato-biliary pathologies are summarized in Table 4. Of the total, 25 patients were found to have pathological conditions with the potential to obstruct the bile duct or to slow bile flow.
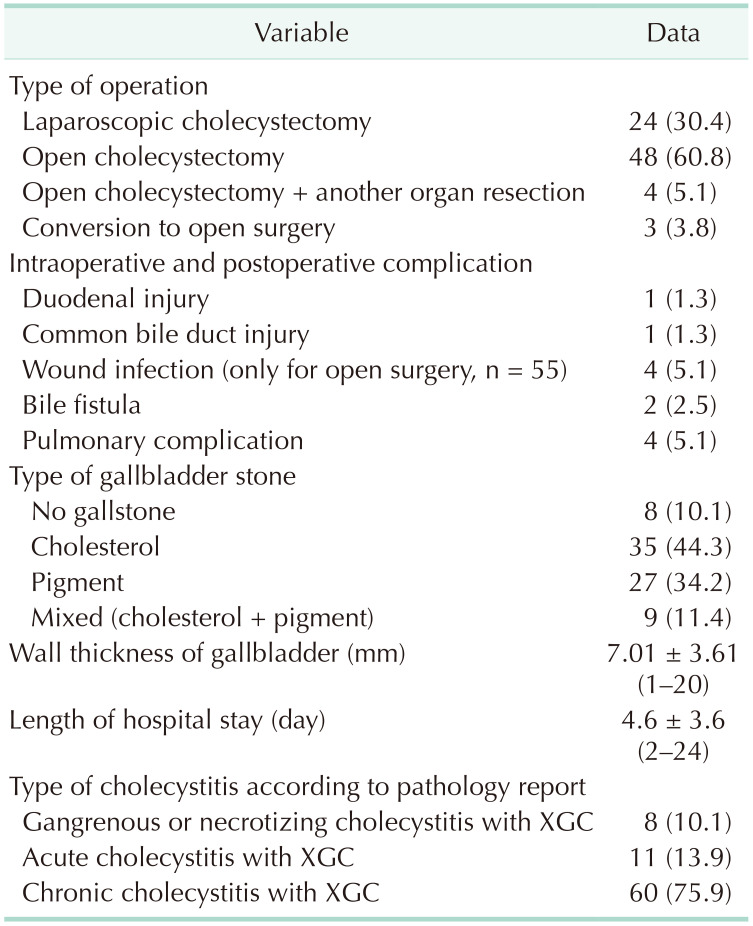
Of 79 patients, 48 (60.8%) underwent open cholecystectomy, 24 (30.4%) underwent LC, 3 (11.1%) underwent conversion to open cholecystectomy due to technical difficulties, and 4 (5.1) underwent cholecystectomy with other organ resections. These patients (n = 4) with XGC underwent procedures involving a cholecystectomy such as pancreaticoduodenectomy for tumor of ampulla of Vater in 1 patient, right hepatectomy for Klatskin tumor in 1 patient, and Roux-en-Y hepaticojejunostomy for cholangiocellular carcinoma in 2 patients, with XGC detected through an examination of cholecystectomy materials. One patient (1.3%) who underwent open surgery experienced a 1-cm perforation in the second part of the duodenum due to adhesions, and this patient underwent primary repair. Furthermore, 1 patient (1.3%) underwent conversion to open cholecystectomy with T-tube placement into the common bile duct after having suffered a common bile duct injury during LC, and this patient was later discharged with full recovery. A minor bile leak developed in one patient who underwent a laparoscopic procedure and in another patient who underwent an open cholecystectomy, and these patients recovered with medical therapy in a mean duration of 5 days. Of the 55 patients who underwent open surgery, 4 (7.3%) developed wound infection that recovered with regular wound dressing changes. A frozen section examination was performed in 20 patients due to suspicion of a tumor by intraoperative macroscopic examination; however, no malignancy was detected in the cases who underwent a frozen section examination. The surgical data of the patients are presented in Table 5. The mean wall thickness of the gallbladder was 7.01 ± 3.61 mm. A pathological examination of the specimens revealed gangrenous or necrotic XGC in 8 patients (10.1%), acute XGC in 11 (13.9%), and chronic XGC in 60 (75.9%).
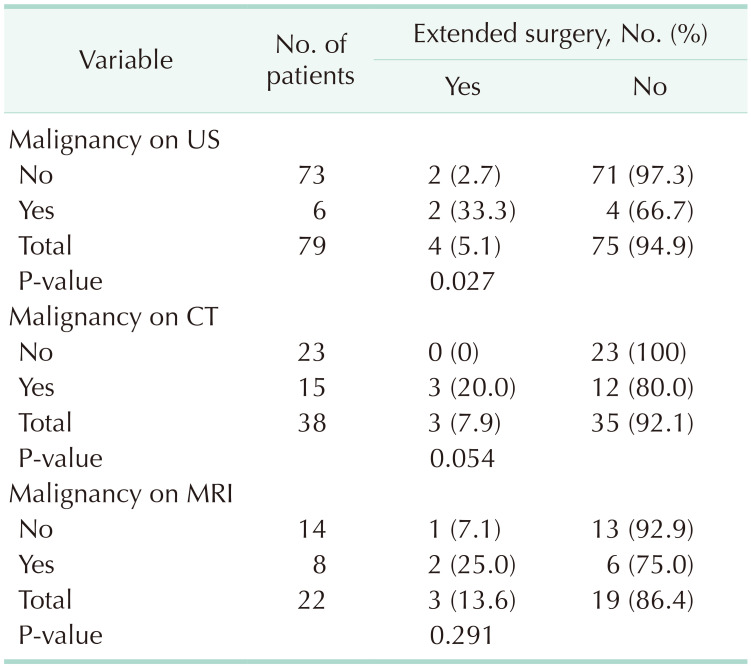
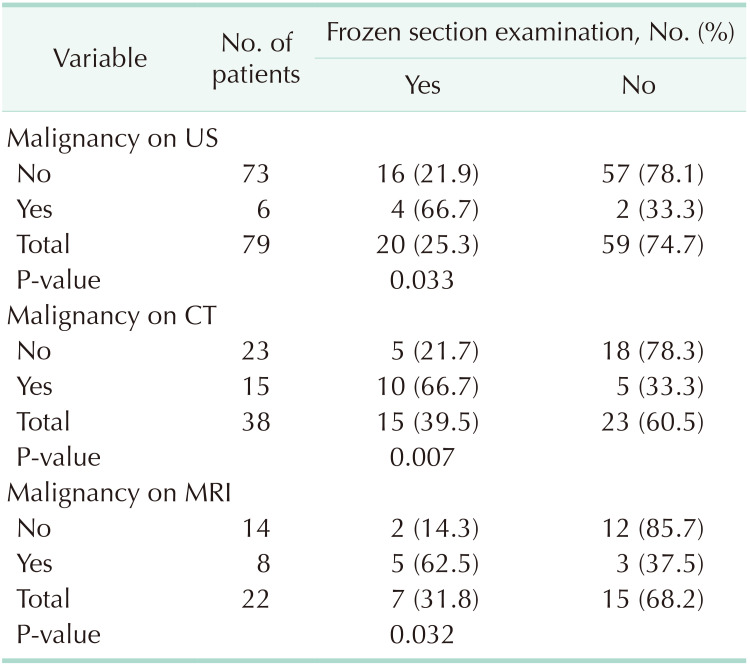
In this study, the term “extended surgery” was used for an operation where more radical surgery was performed than cholecystectomy. Of the 73 patients whose US suggested a benign pathology, 2 underwent extended surgery, and extended surgery was also avoided in patients in whom US suggested a benign pathology (P = 0.027). An increase in the wall thickness of gallbladder, which is an important finding for XGC, was found to be 59.5% (47/79) on US, 81.6% (31/38) on CT, and 81.8% (18/22) on MRI. In the analysis of patients who were evaluated through CT and MRI scans, no statistical difference was found between those who underwent extended surgery vs. those who did not, and it was found that CT and MRI scans did not lead to a result that avoided extended surgery, but rather led to results suggesting a high rate of suspected malignancy (P > 0.05) (Table 6). The rate of frozen section examination was significantly higher among the patients who were found to have malignancy after US, CT, and MRI scans (P < 0.05) (Table 7). No mortality occurred relating to surgery during hospital stay in the postoperative period. No additional pathology or complication occurred in any patient by the 1-year control visit.
This study represents a wide XGC series and radiologically demonstrated that US may be inadequate for demonstration of gallbladder wall thickness for patients pathologically diagnosed with XGC.
XGC is a rare gallbladder disease that is characterized by widespread fibrosis and causes an increase in gallbladder wall thickness [2]. XGC is an increasingly recognized rare variant of chronic cholecystitis characterized by severe proliferative fibrosis and accumulation of lipid-laden macrophages in areas of destructive inflammation [39]. While the former mimics chronic cholecystitis, mass-forming XGC is difficult to differentiate from gallbladder cancer [47].
XGC is a rare special chronic cholecystitis and accounts for 0.7%–13.2% [101112]. In our series of patients, XGC was detected in 0.93% of the cases. There was no clear dominance with regard to gender incidence, with female predominance demonstrated by some authors and male predominance demonstrated by other authors. This is related to low incidence of XGC and lack of clinical data on large samples [1013]. In the present study, XGC was detected in 52 male patients (65.8%) and 27 female patients (34.2%). Previous studies have reported XGC occurring in the 5th and 6th decades [1011], indicating that the chronic inflammatory process is an important factor in the development of this disease. The mean age in the present study was 65.8 ± 14.3 years (range, 36–97 years). In a study by Park et al. [14], the mean age of the patients was 63.2 years, with a predominance of male patients, although there was no significant difference between the number of males and females. Their results related to age and gender characteristics concur with the findings of the present study.
It is worth mentioning that a bibliographic search for this condition occurring at the pediatric age unearthed only 2 articles on the subject, thereby reinforcing the idea of chronicity in the genesis of this disease [1516]. Kim et al. [16] reported a case of 9-year-old patient with XGC without gallstones. To date, not sufficiently addressed in literature, 25 patients (31.6%) in the present study had distal stenosis of the common bile duct, chronic pancreatitis, choledocholithiasis, and other obstructive pathologies that may have caused chronic inflammation in the preoperative period. All patients histopathologically diagnosed with XGC should be further assessed in terms of extrahepatic bile duct obstruction when considering nearly 30% of our patients with XGC had either benign or malign bile duct pathology.
The involvement of the biliary tract as a result of the inflammatory process can also be observed [17]. On the other hand, the absence of intrahepatic biliary dilation is more common in XGC and represents an important finding in the differentiation of gallbladder carcinomas [17]. Goshima et al. [5] reported intrahepatic bile duct dilation in 27.8% and extrahepatic bile duct dilation in 11.1% of patients with XGC, while these rates were 76.5% and 17.6% in patients with gallbladder tumors, respectively. Dilation of the biliary tract was observed in 7 patients (8.9%) in our study. Krishnani et al. [18] reported a significant association between gallbladder carcinoma and XGC (19.6% of cases). Although the exact mechanism underlying the association between the 2 conditions remains unclear, both are considered to be secondary to the chronic inflammatory process caused by cholelithiasis and/or cholecystitis. There have been studies suggesting that XGC is a predisposing factor for gallbladder carcinoma [1920]. No association of XGC and bile duct cancer was observed in this study; a Klatskin tumor in 1 patient, a tumor of ampulla of Vater in 1 patient, and a cholangiocellular carcinoma in 2 patients. When evaluated independently of the gallbladder tumor, an association was found between XGC and tumors of the hepato-pancreato-biliary system, which was observed in 5% of the patients.
The gallbladder wall thickness of 5–9 mm in XGC supports the notion that XGC is a form of chronic cholecystitis [78]. The mean wall thickness of the gallbladder in the present study was 7.01 ± 3.61 mm. Such increases in the wall thickness of the gallbladder may lead to a misdiagnosis of acute cholecystitis as well as tumors, and this may explain the difference between the rate of acute cholecystitis in preoperative imaging studies and the rate of acute cholecystitis in pathology specimens.
The increased presence of tumor markers such as CEA and CA 19-9 should raise suspicion of gallbladder cancer. Some studies have shown that an increased CA 19-9 level (>20 U/mL) had a 79.4% sensitivity and a 79.2% specificity, and an increased CEA level (>4.0 ng/mL) had a 93% specificity, but only a 50% sensitivity [2122]. Chang et al. [23] found that CA 19-9 was commonly measured in the serum of patients with suspected pancreatobiliary malignancy. However, moderate elevations of CA 19-9 (261.5 ± 740.2 U/L) may be seen in patients with benign disease like XGC. CA 19–9 levels were studied in 41 patients in our study, and elevated levels were found in 48.7% of these patients. The highest value was 1,260 U/mL and the second-highest value was 150 U/mL (cut-off value, 37 U/mL). CEA value was not found high in any XGC patient in our study. Cholestatic pathologies involved in the etiology of XGC are also thought to indicate elevated CA 19-9 levels. Although CA 19–9 value may increase in XGC patients, this may be due to the role of obstructive pathologies in the etiology.
Some radiological features have been proposed in patients with thick-walled gallbladder to differentiate XGC from gallbladder cancer; however, reported sensitivity and specificity of these findings to predict XGC in patients with thick-walled gallbladder ranges from 61% to 89% and 65% to 82%, respectively [524]. Also, whether these radiological findings can help in differentiating XGC from gallbladder cancer in patients who have gallbladder mass with or without adjacent organ involvement is not well established. US may help improve the management of XGC and avoid unnecessary radical cholecystectomy [25]. In some previous studies, the features of XGC on US, CT, or MRI are hypoechoic or hypoattenuated nodules in the thickened walls, continuous mucosal lines, and a diffusely thickened gallbladder wall [5]. However, the imaging findings exhibit wide variability [6]. In some previous reports, MRI had the best diagnostic performance among the different imaging modalities [26]. Lee et al. [27] compared the diagnostic performance of these 3 imaging modalities for XGC and found that although the performance of MRI was best, US performed better than CT. In a study by Seo et al. [28], diffuse thickening of the gallbladder wall was observed in 66.6% of the patients, and a rate of gallbladder wall thickening was reported to be 66.6% and 87.7% in further studies [528]. In our study, 4 patients underwent a radical cholecystectomy, making an extended surgery rate of 5%. Of the 73 patients with a benign lesion on US, 2 underwent extended surgery, whereas extended surgery was performed on the patients for whom no malignancy or suspected malignancy was reported on US (P = 0.027). The inability of the radiologist to report characteristic US findings suggestive of XGC, such as soft mucosa and hypoechoic nodules, is considered to be a limitation of the present study. In the present study, an increase in the wall thickness was observed in 81.6% (n = 31) of the patients on CT and in 81.8% (n = 18) of the patients on MRI scans in which possible tumor lesions were reported, but no tumor was detected.
Although LC is the optimum treatment method for benign gallbladder diseases, the laparoscopic approach to the treatment of XGC has been reported to be associated with a high conversion to open cholecystectomy rate, ranging between 10% and 80% [829]. Of the patients who were scheduled for only cholecystectomy in the present study, 48 underwent open cholecystectomy, 24 underwent LC, and 3 underwent conversion to open cholecystectomy, making a total conversion to open cholecystectomy rate of 11.1% (n = 3).
The characteristic findings of XGC may be more prominent in the imaging studies, masking the concurrent adenocarcinoma, and thereby leading to a delay in the diagnosis [25]. No patient in the present study had findings that could have delayed a diagnosis of gallbladder tumor. Some studies have reported that radical surgery options may be considered in cases of high index of suspicion for malignancy, and that the results of frozen section examinations may be misleading [2830]. In suspicious cases, a cholecystectomy specimen must be examined by the operating surgeon and sent for frozen section examination if deemed necessary after inspection and palpation [9]. In the present study, 25.3% (n = 20) of the patients underwent a frozen section examination, which revealed malignancy in none of these patients, and thus extended surgery was avoided.
Higher postoperative complication rates of XGC compared with standard LC have been reported in the literature (13.5–43.5% for XGC vs. 2.6% for standard LC) [1115]. This trend was also observed in our study, in which the overall postoperative complication rate was 15.2% (12/79 patients), and the incidence of complication rate greater than Clavien-Dindo grade III (common bile duct injury and pleural effusion) was 3.8% (3/79 patients). This is a higher morbidity rate than in usual cholecystectomy series (13.5–43.5% for XGC vs. 2.6% for standard LC) [1115].
Gallbladder cancer is a rare disease. XGC, which produces clinical and radiological findings resembling those of gallbladder tumors, must be considered in differential diagnosis to ensure extended surgery is avoided. In the present study, US did not clearly indicate a diagnosis of XGC in all patients, even though it has been considered to be more valuable than CT and MRI scans in differentiating between XGC and malignancy in the published literature. Frozen section examination during surgery can avoid extended surgery and thus reduce morbidity and mortality rates, length of stay, and hospital costs. The finding of an association between XGC and pathologies of the distal common bile duct in the present study indicates a need for prospective randomized controlled trials involving a large number of patients.
The limitations of the present study were that US was dependent on the radiologist who performed it, and that both CT and MRI scans were evaluated by the same radiologist. A potential bias resulting from the fact that more advanced imaging methods were performed in patients with a higher risk of malignancy, both clinically and on US, was also a limitation of this study. The retrospective study design was another limitation of the present study that prevented evaluation of patients during treatment.
In conclusion, XGC is a rare disease that can mimic gallbladder carcinoma. Therefore, it is clear that further imaging methods are needed in the preoperative period other than US. Intraoperative frozen section specimens must be evaluated by experienced pathologists, and the pathologist may guide the surgeon to not perform extended surgery. The definitive diagnosis of XGC depends exclusively on pathologic examination.
References
1. Christensen AH, Ishak KG. Benign tumors and pseudotumors of the gallbladder. Report of 180 cases. Arch Pathol. 1970; 90:423–432. PMID: 4319984.
2. Goodman ZD, Ishak KG. Xanthogranulomatous cholecystitis. Am J Surg Pathol. 1981; 5:653–659. PMID: 7337158.
3. Yang T, Zhang BH, Zhang J, Zhang YJ, Jiang XQ, Wu MC. Surgical treatment of xanthogranulomatous cholecystitis: experience in 33 cases. Hepatobiliary Pancreat Dis Int. 2007; 6:504–508. PMID: 17897914.
4. Sharma D, Babu R, Sood G, Kapoor G, Solanki RS, Thomas S. Xanthogranulomatous cholecystitis masquerading as malignancy with liver metastasis. ANZ J Surg. 2009; 79:946–947. PMID: 20003001.
5. Goshima S, Chang S, Wang JH, Kanematsu M, Bae KT, Federle MP. Xanthogranulomatous cholecystitis: diagnostic performance of CT to differentiate from gallbladder cancer. Eur J Radiol. 2010; 74:e79–e83. PMID: 19446416.
6. Zhao F, Lu PX, Yan SX, Wang GF, Yuan J, Zhang SZ, et al. CT and MR features of xanthogranulomatous cholecystitis: an analysis of consecutive 49 cases. Eur J Radiol. 2013; 82:1391–1397. PMID: 23726123.
7. Levy AD, Murakata LA, Abbott RM, Rohrmann CA Jr. From the archives of the AFIP. Benign tumors and tumorlike lesions of the gallbladder and extrahepatic bile ducts: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2002; 22:387–413. PMID: 11896229.
8. Casas D, Pérez-Andrés R, Jiménez JA, Mariscal A, Cuadras P, Salas M, et al. Xanthogranulomatous cholecystitis: a radiological study of 12 cases and a review of the literature. Abdom Imaging. 1996; 21:456–460. PMID: 8832871.
9. Wang M, Zhang T, Zang L, Lu A, Mao Z, Li J, et al. Surgical treatment for xanthogranulomatous cholecystitis: a report of 74 cases. Surg Laparosc Endosc Percutan Tech. 2009; 19:231–233. PMID: 19542852.
10. Guzmán-Valdivia G. Xanthogranulomatous cholecystitis: 15 years' experience. World J Surg. 2004; 28:254–257. PMID: 14961199.
11. Fligiel S, Lewin KJ. Xanthogranulomatous cholecystitis: case report and review of the literature. Arch Pathol Lab Med. 1982; 106:302–304. PMID: 6896437.
12. Solanki RL, Arora HL, Gaur SK, Anand VK, Gupta R. Xanthogranulomatous cholecystitis (XGC): a clinicopathological study of 21 cases. Indian J Pathol Microbiol. 1989; 32:256–260. PMID: 2632411.
13. Han SH, Chen YL. Diagnosis and treatment of xanthogranulomatous cholecystitis: a report of 39 cases. Cell Biochem Biophys. 2012; 64:131–135. PMID: 22707297.
14. Park JW, Kim KH, Kim SJ, Lee SK. Xanthogranulomatous cholecystitis: is an initial laparoscopic approach feasible? Surg Endosc. 2017; 31:5289–5294. PMID: 28593410.
15. Kawana T, Suita S, Arima T, Hirayama Y, Ishii K, Minamishima I, et al. Xanthogranulomatous cholecystitis in an infant with obstructive jaundice. Eur J Pediatr. 1990; 149:765–767. PMID: 2121491.
16. Kim H, Cho Y, Park J. Xanthogranulomatous cholecystitis not associated with gallstone in a 9-year-old girl. J Korean Surg Soc. 2009; 77:72–74.
17. Goldar-Najafi A, Khettry U. Xanthogranulomatous choledochitis: a previously undescribed mass lesion of the hepatobiliary and ampullary region. Semin Liver Dis. 2003; 23:101–106. PMID: 12616455.
18. Krishnani N, Shukla S, Jain M, Pandey R, Gupta RK. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000; 44:508–514. PMID: 10934941.
19. Kocaöz S, Turan G. Preneoplastic and neoplastic gallbladder lesions detected after cholecystectomy. Prz Gastroenterol. 2019; 14:193–197. PMID: 31649791.
20. Hale MD, Roberts KJ, Hodson J, Scott N, Sheridan M, Toogood GJ. Xanthogranulomatous cholecystitis: a European and global perspective. HPB (Oxford). 2014; 16:448–458. PMID: 23991684.
21. Misra S, Chaturvedi A, Misra NC, Sharma ID. Carcinoma of the gallbladder. Lancet Oncol. 2003; 4:167–176. PMID: 12623362.
22. Strom BL, Maislin G, West SL, Atkinson B, Herlyn M, Saul S, et al. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990; 45:821–824. PMID: 2335386.
23. Chang BJ, Kim SH, Park HY, Lim SW, Kim J, Lee KH, et al. Distinguishing xanthogranulomatous cholecystitis from the wall-thickening type of early-stage gallbladder cancer. Gut Liver. 2010; 4:518–523. PMID: 21253302.
24. Uchiyama K, Ozawa S, Ueno M, Hayami S, Hirono S, Ina S, et al. Xanthogranulomatous cholecystitis: the use of preoperative CT findings to differentiate it from gallbladder carcinoma. J Hepatobiliary Pancreat Surg. 2009; 16:333–338. PMID: 19280109.
25. Zhang F, Chen W, Zhang L, Hou C, Zhang M. Usefulness of ultrasound in differentiating xanthogranulomatous cholecystitis from gallbladder carcinoma. Ultrasound Med Biol. 2019; 45:2925–2931. PMID: 31447238.
26. Kang TW, Kim SH, Park HJ, Lim S, Jang KM, Choi D, et al. Differentiating xanthogranulomatous cholecystitis from wall-thickening type of gallbladder cancer: added value of diffusion-weighted MRI. Clin Radiol. 2013; 68:992–1001. PMID: 23622795.
27. Lee ES, Kim JH, Joo I, Lee JY, Han JK, Choi BI. Xanthogranulomatous cholecystitis: diagnostic performance of US, CT, and MRI for differentiation from gallbladder carcinoma. Abdom Imaging. 2015; 40:2281–2292. PMID: 25952571.
28. Seo SH, Park JI, Kim JS, Kim KH, Choi CS, Choi YK. Xanthogranulomatous cholecystitis: a retrospective analysis of 36 cases. J Korean Surg Soc. 2009; 76:371–377.
29. Kim JH, Jeong IH, Yoo BM, Kim JH, Kim MW, Kim WH. Is xanthogranulomatous cholecystitis the most difficult for laparoscopic cholecystectomy? Hepatogastroenterology. 2009; 56:597–601. PMID: 19621662.
30. Benbow EW. Xanthogranulomatous cholecystitis. Br J Surg. 1990; 77:255–256. PMID: 2182176.
Fig. 1
The ultrasonography (US), CT, and MRI findings. (A) The US revealed that there were irregular gallbladder wall thickening and multiple millimetric gallstones (arrow) were noted in the neck of gallbladder. (B) The CT showed that the gallbladder wall was thickened and had an edematous appearance, and soft tissue lesions (arrows) that took up space in the gallbladder region attracted attention. (C) The MRI demonstrated that the gallbladder was larger than normal, and thickening of the gallbladder wall, soft tissue density in the gallbladder region, and mixed space-occupying lesions (arrows) in the gallbladder lumen were also observed.
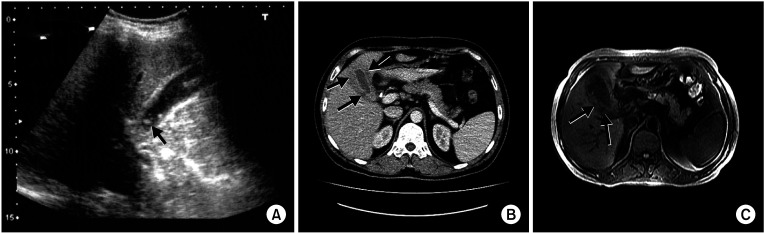
Fig. 2
Macroscopic appearance of the excised gallbladder with xanthogranulomatous cholecystitis. There were a lot of grayish-yellow nodules in the gallbladder wall upon opening of the gallbladder.
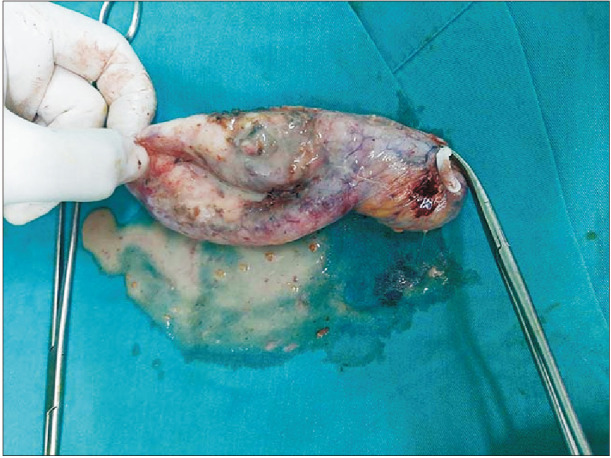
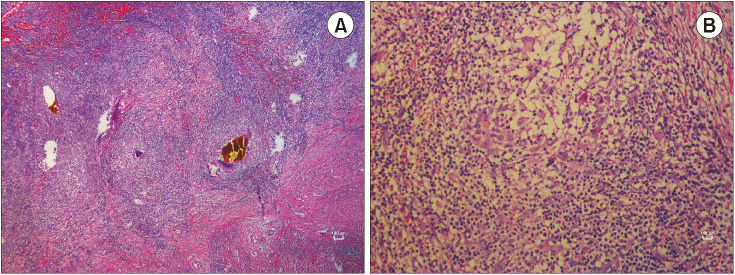
Fig. 3
Histopathological images. (A) Intense histiocytic and lymphocytic infiltration with occasional bile pigments (H&E staining, ×40). (B) The structure of a granuloma is formed by multinucleated giant cells and lymphoplasmacytic infiltration (H&E staining, ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download