Abstract
Purpose
The frequency of iatrogenic femoral artery pseudoaneurysm (FAP) diagnoses has recently increased due to the growing use of diagnostic and interventional procedures involving large diameter sheaths, as well as more potent anticoagulation procedures. In this study, we aimed to present our experience with ultrasound-guided thrombin injection (UGTI) in patients with iatrogenic FAP.
Methods
We studied patients with FAP who were under anticoagulant or antiplatelet therapies preoperatively, or who had received a loading dose during an interventional procedure. The outcomes of patients with FAP treated with UGTI were compared with those of patients who underwent open surgical repair for pseudoaneurysms.
Results
Among the 55 patients included in this study, 24 had UGTI while 31 had open surgery. The success rate was 95.8% when taking into consideration primary and secondary attempts. The mean duration of the procedure was shorter in patients with UGTI (10.1 ± 3.54 minutes) when compared with those who underwent open surgery (76.55 ± 26.74 minutes, P ≤ 0.001). In addition, the total complication frequency was significantly higher in the open surgery group (P = 0.005), as was their length of hospital stay (P < 0.001). Cost analysis showed significant differences between UGTI ($227.50 ± $82.90) and open surgery ($471.20 ± $437.60, P = 0.01).
Iatrogenic femoral artery pseudoaneurysm (FAP), which is related to the femoral arterial puncture site, is one of the most common vascular complications of cardiac and peripheral angiographic procedures [123]. The frequency of FAP diagnoses has recently increased due to the growing use of diagnostic and interventional procedures involving large diameter sheaths, as well as more potent anticoagulation procedures [456]. FAP can be observed in 0.2%–5% of cases following diagnostic procedures which can further increase among interventional procedures [1789].
Pseudoaneurysm is formed when the femoral arterial puncture site fails to seal, resulting in arterial rupture into the surrounding tissues, which eventually forms a pulsatile hematoma. These lesions do not contain a fibrous wall and are surrounded by a shell of hematoma and the pressure of surrounding soft tissues [178]. Factors associated with the formation of the pseudoaneurysm are the use of antiplatelet agents, anticoagulation, large sheath size, older age, obesity, ineffective periprocedural compression, catheterization of artery and vein at the same procedure, hypertension, peripheral arterial disease, hemodialysis, and other complex interventions [3]. Doppler ultrasound is the current gold standard method for the diagnosing of FAP. One of the most serious complications involves a rupture and bleeding, while pain, progressive enlargement, distal embolization, skin necrosis, deep venous thrombosis, neuropathy, and distal limb ischemia may also be observed [510].
Watchful waiting for spontaneous closure is appropriate for asymptomatic patients with small FAPs (<2 cm) [3]. However, untreated large FAPs (≥2 cm) may cause complications such as rupture, deep vein thrombosis, neuropathy or skin necrosis [31112]. Surgical treatment remains the gold standard treatment for iatrogenic FAPs that do not spontaneously thrombose. However, in the early 1990s, ultrasound-guided thrombin injection (UGTI) was introduced as a new technique for the management of iatrogenic FAP. This novel tool was safer, quicker and less painful than conventional surgical treatment [613].
In this study, we aimed to present our experience with UGTI in patients with iatrogenic FAP who use anticoagulants, in terms of safety and efficiency and compare the results with open surgery.
This study was performed in a high-volume training and research hospital in which interventional and diagnostic cardiac and peripheral procedures are performed daily. The study was approved by the Clinical Research Ethical Committee of Tepecik Training and Research Hospital, with an approval number of 2018/13-12. Between April 2012 and April 2018, 19.375 cardiac and 3.711 percutaneous endovascular peripheral and neuroradiologic interventions were performed in our hospital. Of these patients, 324 cases of FAP were diagnosed with an incidence of 1.4%. In our retrospective study, we investigated patients with iatrogenic FAP who were under anticoagulation or antiplatelet preoperatively or who have received a loading dose during an interventional procedure. A total of 55 patients were included in this study. Informed consent obtained from all patients. The outcomes of 24 patients with FAP and treated with UGTI were compared with the results of 31 patients who underwent open surgical repair for pseudoaneurysm. All procedures and follow-ups were conducted by cardiovascular surgeons in a cardiovascular surgery department.
Our general approach to patients with iatrogenic FAP is summarized in Fig. 1. Patients with asymptomatic FAP with a diameter <2 cm are observed for spontaneous closure for a duration of 4 weeks. For patients in whom no spontaneous closure was observed after 4 weeks, or whose FAP is ≥2 cm in diameter, and/or are symptomatic, the decision for intervention was made according to anticoagulation or antiplatelet status. Prior to 2017 our approach to FAP in patients given anticoagulation or antiplatelet as a loading dose or who had been undergoing anticoagulation or antiplatelet interventions previously was to perform open surgery. After 2017, we updated our approach to UGTI as a first-line treatment option for these patients. In individuals with no history of anticoagulation or antiplatelet interventions nor complications from FAP, ultrasound-guided compression therapy (UGCT) is the first-line treatment. If UGCT fails, UGTI is recommended. If UGTI fails despite 2 attempts in 48 hours, surgery is then recommended. In addition, open surgery is the initial treatment option for patients with complicated FAPs.
Our inclusion criteria accepted patients >18 years of age with iatrogenic noncomplicated symptomatic FAPs and who were under anticoagulation or antiplatelet therapy. We excluded patients <18 years of age and whose FAPs arose from sites other than the groin. Also, FAPs that are infected or with preprocedural skin ulcerations, necrosis, or arteriovenous fistulas were defined as complicated FAPs and excluded from the trial. In addition, we excluded failed UGCT cases as they were not under anticoagulation or antiaggregation therapy preprocedurally. Asymptomatic patients were also excluded; however, we did include asymptomatic patients who later became symptomatic during follow-up. Patients with traumatic pseudoaneurysms were excluded from our study as they may have needed surgical management.
Pseudoaneurysm was suspected in patients with a history of femoral artery catheterization; when pain, ecchymosis, a pulsatile mass, necrotic skin changes, or skin infection occurred in the intervention area. Pseudoaneurysm diagnoses were made using color Doppler ultrasonography (CDU) (Philips Epiq 5 ultrasound with L12-4 12-4 MHz linear probe, Philips Healthcare, Amsterdam, Netherlands). The area of pseudoaneurysm sac (multiplied by the 2 dimensions), as well as the width and length (from FAP to the vessel) of the FAP were recorded. Each examination was performed by an experienced physician. The ultrasonographic criteria for diagnosis involved the observation of swirling color flow in a mass separate from the affected artery, color flow within a track leading from the artery to the mass that is consistent with the pseudoaneurysm neck, and a typical to-and-fro Doppler waveform in the pseudoaneurysm neck [14]. The flow lumen diameter was optimized to ensure proper acquirement of pseudoaneurysm size, while the neck length was measured from the pseudoaneurysm to the vessel of origin. The width of the neck was also measured.
Physical examination was performed on all patients, while lower extremity pulses were noted. If there was a history of peripheral arterial disease, or in case of pulselessness, arterial CDU was performed for peri-interventional and postinterventional evaluation.
Informed consent was obtained from all patients who underwent UGTI and open surgery. Open surgery was performed using local anesthesia, sedation, or general anesthesia. In all patients who underwent open surgery, primary repair with 6.0 polyproline sutures was performed under sterile conditions in the operation room.
In patients who underwent UGTI, human thrombin was obtained for the management of local bleeding surrounding the vascular access site and was diluted in physiological serum with calcium chloride (1 mL = 500 U). The affected groin was cleaned with povidone-iodine and covered with a sterile cover. Under ultrasound guidance and without local anesthesia, a 21-G needle was used to puncture into the pseudoaneurysm sac and the needle was positioned away from the pseudoaneurysm neck. The appearance of sac and flow was checked consistently with CDU when slow injection of thrombin was performed. The injection was continued until complete obliteration of the sac was achieved. Intervention success was described for complete thrombosis of the FAP without any sac flow under CDU. If most of the sac became thrombosed but flow within the pseudoaneurysm neck did not cease, we waited for 24 hours rather than opting for an immediate repeat of the injection. Recurrence of FAP was described as active blood flow into the sac after 24 hours of successful treatment. Under these circumstances, additional thrombin injection was performed as described. Complications of UGTI such as thromboembolic and allergic events were also noted. Follow-up was performed on all patients within the first 12–24 hours, 1st week and 1st month.
Patient demographics, type and indication of catheterization (cardiac diagnostic, cardiac interventional, peripheral vascular diagnostic, peripheral vascular interventional), sheath size, access site, anticoagulation or antiplatelet status, intraoperative complications, pseudoaneurysm dimensions, and follow-up were reviewed from medical records.
Statistical analysis was performed using IBM SPSS Statistics ver. 20.0 (IBM Co., Armonk, NY, USA). Numeric variables were summarized as mean ± standard deviation values. Categorical variables were evaluated with cross-table analysis and depicted numerically. Student t-test was used for normally distributed data measured on a continuous/interval scale, while Mann-Whitney U test was used for nonnormally distributed data. Pearson correlation test was used to measure the statistical relationships or associations between continuous variables. P < 0.05 was considered statistically significant.
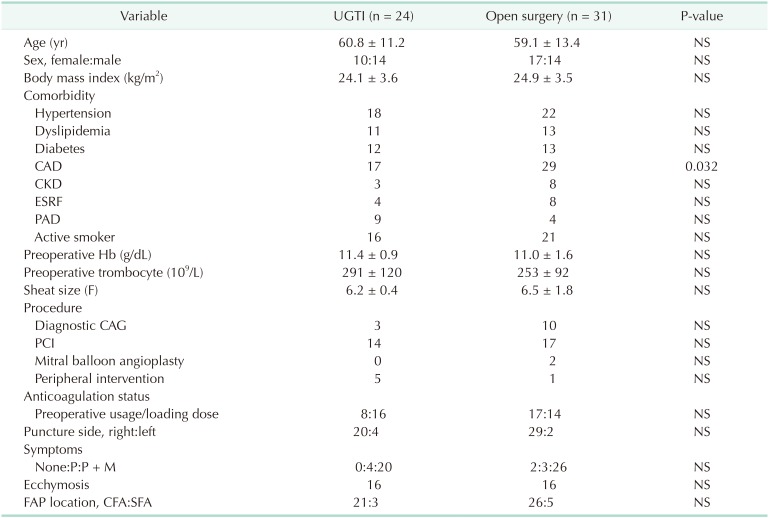
Patient demographics, cardiovascular risk factors, preoperative hemoglobin (Hb) and thrombocyte values are presented in Table 1. Among the 55 patients included in this study, 24 underwent UGTI (10 females, 14 males), while 31 (17 females, 14 males) underwent open surgery. There was no statistically significant difference between groups in terms of age, sex distribution, body mass index values or comorbidities. The only exception was for coronary artery disease, which was observed to be higher in patients who underwent open surgery (P = 0.032). Preoperative Hb and thrombocyte values were also similar between groups. In addition, there was no statistically significant difference between groups in terms of the type of procedure that caused FAP, nor the sheath size used for the procedure (Table 1). All patients were under anticoagulant or antiplatelet therapy, with individual patient status summarized in Table 1. The patients admitted with pain with or without accompanying mass formation was defined as symptomatic patients who formed the majority of the whole data (UGTI group, 24 of 24 patients; surgery group, 29 of 31 patients). Only 2 asymptomatic patients were included to the surgery group during their follow-up due to increase in FAP diameters and becoming symptomatic. The right femoral artery remained the puncture site for most patients. The origin of FAP most commonly originated from the common femoral artery, while few derived from the superficial femoral artery. There was no significant difference between patients who underwent UGTI or open surgery with regards to these parameters.
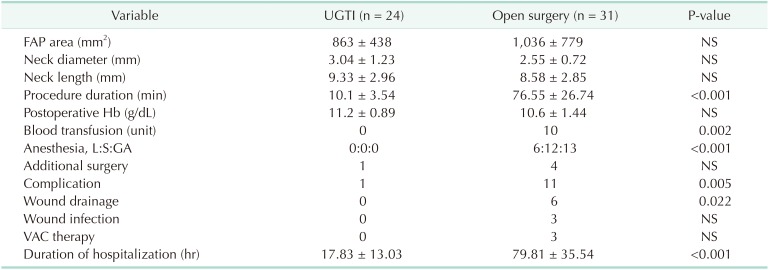
Procedure-related data are summarized in Table 2. FAP area, neck length and neck diameter were similar between the 2 groups. In our study, UGTI was primarily successful in 18 of 24 (75%) patients. Five patients (20.8%) needed additional thrombin injection in the first 24 hours, all of which were successfully performed. The mean thrombin amount used for patients who underwent UGTI was 1.31 ± 0.58 mL. Among the 18 patients who experienced successful UGTI in the first attempt, the mean thrombin dose was 0.14 mL/cm2; while in the 5 patients with a failed first attempt at UGTI, the mean thrombin dose was 0.13 mL/cm2. Thrombin doses in first attempts were similar in all patients. One patient (4.2%) underwent prompt surgical intervention due to thrombin emboli, which was accepted as a failure for UGTI treatment. None of the patients had FAP recurrence. Therefore, where primary and secondary attempts are concerned, the success rate was 95.8%.
Mean duration of the procedure were significantly shorter in patients who underwent UGTI (10.1 ± 3.54 minutes) when compared with patients who sustained open surgery (76.55 ± 26.74 minutes, P ≤ 0.001). Ten patients who underwent open surgery needed a blood transfusion while none of the patients who had UGTI received transfusion (P = 0.002). None of the patients in the UGTI group received anesthesia while all patients received local anesthesia, sedation, or general anesthesia in the open surgery group (P < 0.001). Patients in the open surgery group also experienced more complications than patients in the UGTI group (P = 0.005) (Table 2). One patient who underwent UGTI needed additional surgery under emergency conditions due to acute thrombin emboli, which was treated successfully with embolectomy. On the other hand, 4 patients in the open surgery group needed additional surgeries for hematoma drainage. Wound complications were observed in 11 patients in the open surgery group, 2 of which only had maceration, 6 of whom needed wound drainage, and 3 of whom had infected wounds with a large healing defect that needed vacuum-assisted closure therapy. None of the patients in the UGTI group experienced wound complications. Total complication frequency and need for wound drainage was significantly higher in the open surgery group (P = 0.005 and P = 0.022, respectively). However, no statistically significant correlation between end-stage renal failure (ESRF) and complication risk was found among patients. Upon closer inspection, we observed that the occurrence of ESRF was found to be significantly associated with increased complications in the open surgery group (P = 0.005). Length of hospital stay was also significantly higher in patients who underwent open surgery (79.81 ± 35.54 hours) when compared to the UGTI group (17.83 ± 13.03 hours, P < 0.001).
Cost analysis showed significant differences between UGTI and open surgery groups, with the mean cost for UGTI being $227.50 ± $82.90, while the open surgery cost $471.20 ± $437.60 (P = 0.01).
In this present study, we have found that UGTI is a safe and effective method for the treatment of iatrogenic FAPs, which entails lower complication risks, shorter hospital stays, lower cost, less wound infection and better wound healing when compared with open surgery. This is the first study, to the best of our knowledge, that compares the outcomes of UGTI with open surgery.
Until the early 1990s, surgical treatment remained the gold standard for iatrogenic FAPs that do not spontaneously thrombose. Thereafter, UGCT and UGTI for the management of iatrogenic FAP were introduced as new treatment options [61013]. Endovascular treatment with covered stents and coil embolization is another alternative option that is mainly used for the treatment of FAPs. In addition, the use of closure devices is a novel technique for FAP treatment [15]. All approaches have their distinct advantages and disadvantages. In our study, we compared outcomes of UGTI and open surgery. We did not involve patients who underwent UGCT, as all our patients were under anticoagulant or antiplatelet therapy and UGCT is known to have higher failure rates that reach 70% in patients under such therapies [14].
In our study, the success rate was 95.8% when both primary and secondary attempts are accepted. Through their meta-analysis that included 318 patients, Kontopodis et al. [16] found the success rate of UGTI to be 97.4%, which is similar to our findings. It should be noted that these are early experiences in our center and 3 out of 5 patients that needed additional thrombin doses experienced interventions in the first 6 months of our involvement. This could partly explain the reason for needing additional thrombin injections. That said, there was no statistically significant difference between thrombin doses in 23 patients with successful UGTI in their first or second attempts. Another reason for needing a second attempt in some of the patients despite giving similar doses may also be explained by differences in the protein C and thrombomodulin systems of the patients. Thrombin and thrombomodulin complex activates protein C, which then prompts the coagulation system to reduce the relative number and size of mature thrombi, resulting in the resolution of thrombus in the FAP [1718]. Even with successful treatment, recurrence may occur after 24 hours of follow-up. However, in our study, none of the patients had FAP recurrence after a successful intervention.
Mean duration of the procedure was significantly shorter in patients who underwent UGTI. As all patients in our study received anticoagulant or antiplatelet therapy, bleeding control may be adversely affected in these patients, potentially contributing to the increased duration of surgical procedure.
UGTI can be performed with or without local anesthesia [1920]. In our study, none of the patients in the UGTI group needed any kind of anesthesia including analgesia and all procedures were well tolerated by the patients. However, in the open surgery group, all patients received anesthesia, which may increase the risks of the surgical procedure due to complications. Fortunately, we did not encounter any cardiac complications. However, due to a high incidence of coronary artery disease among patients with FAP, risks caused by anesthesia may be even more increased. Therefore, patients in the UGTI group are protected from those risks.
The only complication we have seen was thrombin emboli which was accepted as a failure of the UGTI treatment. The patient developed progressive acute ischemia of the lower limb and underwent prompt surgical intervention with successful embolectomy and primary repair of the FAP. The sac area of the patient was 0.28 cm2 while the neck of the sac was short (0.3 cm) and wide (0.9 cm). Altogether, this was one of the smallest sac areas among the included patients. It is known that thrombin emboli risk may increase as the sac area and neck length decrease while the neck width increases [21]. In opposition to this view, the study conducted by Yang et al. [19] showed that FAP neck dimensions did not affect treatment efficiency. Kontopodis et al. [16] also showed that UGTI was associated with a very low complication risk of 0.68%. In their meta-analysis of data regarding complication rates of 292 patients with UGTI, they found only 2 cases with complications. One of them had peripheral emboli while another case had skin necrosis. The reason for the relatively higher complication incidence of our study may be the relatively small sample size. Pezzullo and Cronan [22] encountered one complication of emboli in their study with 23 patients who underwent UGTI, which is a complication rate of 4.35%, and is similar to ours. Skin infection, which is a potential complication of UGTI, was also observed in one patient from Weinmann et al. [23]. Another important and fatal but rare complication of UGTI is an anaphylactic reaction to thrombin. Therefore, 2 types of thrombin preparations are being used for this method as the use of bovine thrombin and repeated exposure to thrombin is associated with a higher risk of allergic and anaphylactic reactions [24].
Although surgery is the traditional approach, it is a relatively expensive procedure with higher risks concerning anesthesia, poor healing of surgical site, prolonged hospitalization, delayed ambulation, higher risks of complications such as bleeding, neuralgia, and death [14]. According to our study; open surgery was found to be associated with higher complication rates. When we took a closer look at open surgery groups, we found that having ESRF was significantly associated with increased complication risk. However, this was not valid for patients in the UGTI group. This outcome could be explained by the already damaged hemostatic mechanisms in patients with ESRF who are in need of anticoagulant or antiplatelet therapies that cause further deterioration. As all patients in our study were under anticoagulation or antiplatelet therapy, this deterioration of coagulation may have increased the perioperative and postoperative risks of bleeding and developing hematoma. Wound hematomas and seromas can lead to maceration and edge separation, which can adversely affect wound healing and cause wound infections.
According to our study, UGTI is a less expensive option compared to open surgery. Prolonged hospitalization, high complication rates, and need for anesthesia may explain this significant difference. Ten patients in the open surgery group needed a blood transfusion, while none of the patients in the UGTI group received any blood transfusion. None of the patients who received blood transfusion encountered any complications. However, increased need for blood transfusion may adversely affect the cost and contribute to additional burden on patients, especially on those suffering from ESRF.
The small number of patients limits the power of our study and exaggerates our complication results. The generalizability of our study is also limited by the small sample size that consisted of patients from a single center, as well as its retrospective and nonrandomized nature.
In conclusion, we have found that UGTI is a fast learned, safer and more cost-effective choice of treatment compared with surgery, justifying it as a first approach method of management for FAP. In our series, anticoagulation status did not impact recurrence rates. However, UGTI treatment is still an off-label use of thrombin and further randomized controlled trials are needed for UGTI to become the standard treatment for FAP.
References
1. Katzenschlager R, Ugurluoglu A, Ahmadi A, Hulsmann M, Koppensteiner R, Larch E, et al. Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology. 1995; 195:463–466. PMID: 7724767.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981; 138:273–281. PMID: 7455105.
3. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007; 115:2666–2674. PMID: 17515479.
4. Coley BD, Roberts AC, Fellmeth BD, Valji K, Bookstein JJ, Hye RJ. Postangiographic femoral artery pseudoaneurysms: further experience with US-guided compression repair. Radiology. 1995; 194:307–311. PMID: 7824703.
5. O'Sullivan GJ, Ray SA, Lewis JS, Lopez AJ, Powell BW, Moss AH, et al. A review of alternative approaches in the management of iatrogenic femoral pseudoaneurysms. Ann R Coll Surg Engl. 1999; 81:226–234. PMID: 10615187.
6. Kang SS, Labropoulos N, Mansour MA, Baker WH. Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg. 1998; 27:1032–1038. PMID: 9652465.
7. Kresowik TF, Khoury MD, Miller BV, Winniford MD, Shamma AR, Sharp WJ, et al. A prospective study of the incidence and natural history of femoral vascular complications after percutaneous transluminal coronary angioplasty. J Vasc Surg. 1991; 13:328–333. PMID: 1990173.
8. Houlind K, Jepsen JM, Saicu C, Vammen S, Christensen JK, Ravn H. Current management of inguinal false aneurysms. J Cardiovasc Surg (Torino). 2017; 58:278–283.
9. Eleshra A, Kim D, Park HS, Lee T. Access site pseudoaneurysms after endovascular intervention for peripheral arterial diseases. Ann Surg Treat Res. 2019; 96:305–312. PMID: 31183335.
10. Fellmeth BD, Roberts AC, Bookstein JJ, Freischlag JA, Forsythe JR, Buckner NK, et al. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology. 1991; 178:671–675. PMID: 1994400.
11. Toursarkissian B, Allen BT, Petrinec D, Thompson RW, Rubin BG, Reilly JM, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997; 25:803–808. PMID: 9152307.
12. Kent KC, McArdle CR, Kennedy B, Baim DS, Anninos E, Skillman JJ. A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J Vasc Surg. 1993; 17:125–131. PMID: 8421328.
13. Cope C, Zeit R. Coagulation of aneurysms by direct percutaneous thrombin injection. AJR Am J Roentgenol. 1986; 147:383–387. PMID: 3487958.
14. Eisenberg L, Paulson EK, Kliewer MA, Hudson MP, DeLong DM, Carroll BA. Sonographically guided compression repair of pseudoaneurysms: further experience from a single institution. AJR Am J Roentgenol. 1999; 173:1567–1573. PMID: 10584803.
15. Robken J, Shammas NW. Novel technique to treat common femoral artery pseudoaneurysm using angio-seal closure device. Int J Angiol. 2016; 25:266–270. PMID: 27867294.
16. Kontopodis N, Tsetis D, Tavlas E, Dedes A, Ioannou CV. Ultrasound guided compression versus ultrasound guided thrombin injection for the treatment of post-catheterization femoral pseudoaneurysms: systematic review and meta-analysis of comparative studies. Eur J Vasc Endovasc Surg. 2016; 51:815–823. PMID: 27026390.
17. Sadler JE. Thrombomodulin structure and function. Thromb Haemost. 1997; 78:392–395. PMID: 9198185.
18. Gruber A, Mori E, del Zoppo GJ, Waxman L, Griffin JH. Alteration of fibrin network by activated protein C. Blood. 1994; 83:2541–2548. PMID: 8167339.
19. Yang EY, Tabbara MM, Sanchez PG, Abi-Chaker AM, Patel J, Bornak A, et al. Comparison of ultrasound-guided thrombin inject ion of iat rogenic pseudoaneurysms based on neck dimension. Ann Vasc Surg. 2018; 121–127. PMID: 28887253.
20. Kuma S, Morisaki K, Kodama A, Guntani A, Fukunaga R, Soga Y, et al. Ultrasound-guided percutaneous thrombin injection for post-catheterization pseudoaneurysm. Circ J. 2015; 79:1277–1281. PMID: 25797019.
21. Hashemi Fard O. Iatrogenic femoral artery pseudoaneurysm (review of treatment options). ARYA Atheroscler. 2010; 6:74–77. PMID: 22577418.
22. Pezzullo JA, Cronan JJ. Postcatheterization pseudoaneurysms: new developments in the diagnosis and treatment with ultrasound. Ultrasound Q. 2001; 17:227–234. PMID: 12973063.
23. Weinmann EE, Chayen D, Kobzantzev ZV, Zaretsky M, Bass A. Treatment of postcatheterisation false aneurysms: ultrasound-guided compression vs ultrasound-guided thrombin injection. Eur J Vasc Endovasc Surg. 2002; 23:68–72. PMID: 11748951.
24. Jalaeian H, Misselt A. Anaphylactic reac t ion to bovine thrombi n in ultrasound-guided treatment of femoral pseudoaneurysm. J Vasc Interv Radiol. 2015; 26:915–916. PMID: 26003458.
Fig. 1
Flow diagram of the treatment protocol for iatrogenic femoral artery pseudoaneurysms. FAP, femoral artery pseudoaneurysm; UGTI, ultrasound-guided thrombin injection.
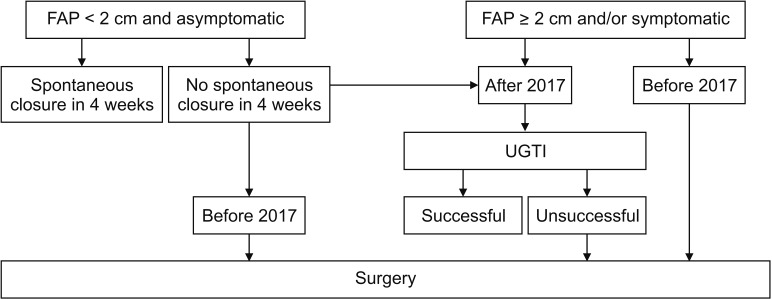
Table 1
Demographic features, cardiovascular risk factors, and procedural characteristics of patients having UGTI and open surgery
Values are presented as mean ± standard deviation or number.
UGTI, ultrasound-guided thrombin injection; CAD, coronary artery disease; CKD, chronic kidney disease; ESRF, end-stage renal failure; PAD, peripheral artery disease; Hb, hemoglobin; F, French; CAG, coronary angiography; PCI, percutaneous coronary intervention; P, pain; M, mass; FAP, femoral artery pseudoaneurysm; CFA, common femoral artery; SFA, superficial femoral artery; NS, nonsignificant.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download