1. van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013; 14:210–218. PMID:
23395398.

2. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005; 6:477–484. PMID:
15992696.
3. Mavros MN, de Jong M, Dogeas E, Hyder O, Pawlik TM. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg. 2013; 100:711–718. PMID:
23364914.

4. Yamashita S, Sheth RA, Niekamp AS, Aloia TA, Chun YS, Lee JE, et al. Comprehensive complication index predicts cancer-specific survival after resection of colorectal metastases independent of RAS mutational status. Ann Surg. 2017; 266:1045–1054. PMID:
27735824.

5. Liu VX, Rosas E, Hwang J, Cain E, Foss-Durant A, Clopp M, et al. Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg. 2017; 152:e171032. PMID:
28492816.

6. Kim MK, Kim JG, Lee G, Won DD, Lee YS, Kye BH, et al. Comparison of the effects of an ERAS program and a single-port laparoscopic surgery on postoperative outcomes of colon cancer patients. Sci Rep. 2019; 9:11998. PMID:
31427651.

7. Hughes MJ, Hackney RJ, Lamb PJ, Wigmore SJ, Christopher Deans DA, Skipworth RJ. Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg. 2019; 43:1661–1668. PMID:
30788536.

8. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID:
19638912.
9. Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013; 258:1–7. PMID:
23728278.
10. Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013; 56:667–678. PMID:
23575408.
11. Feng F, Li XH, Shi H, Wu GS, Zhang HW, Liu XN, et al. Fast-track surgery combined with laparoscopy could improve postoperative recovery of low-risk rectal cancer patients: a randomized controlled clinical trial. J Dig Dis. 2014; 15:306–313. PMID:
24597608.

12. Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014; 38:1531–1541. PMID:
24368573.

13. Lee CS, Won DD, Oh SN, Lee YS, Lee IK, Kim IH, et al. Prognostic role of pre-sarcopenia and body composition with long-term outcomes in obstructive colorectal cancer: a retrospective cohort study. World J Surg Oncol. 2020; 18:230. PMID:
32859211.

14. Verheyden C, Orliac C, Millet I, Taourel P. Large-bowel obstruction: CT findings, pitfalls, tips and tricks. Eur J Radiol. 2020; 130:109155. PMID:
32711335.

15. Casadei-Gardini A, Scarpi E, Ulivi P, Palladino MA, Accettura C, Bernardini I, et al. Prognostic role of a new inflammatory index with neutrophil-to-lymphocyte ratio and lactate dehydrogenase (CII: Colon Inflammatory Index) in patients with metastatic colorectal cancer: results from the randomized Italian Trial in Advanced Colorectal Cancer (ITACa) study. Cancer Manag Res. 2019; 11:4357–4369. PMID:
31191000.
16. Mazaki J, Katsumata K, Kasahara K, Tago T, Wada T, Kuwabara H, et al. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020; 20:922. PMID:
32977767.

17. Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010; 147:339–351. PMID:
20004450.

18. Kim TH, Suh YS, Huh YJ, Son YG, Park JH, Yang JY, et al. The comprehensive complication index (CCI) is a more sensitive complication index than the conventional Clavien-Dindo classification in radical gastric cancer surgery. Gastric Cancer. 2018; 21:171–181. PMID:
28597328.

19. Tu RH, Lin JX, Li P, Xie JW, Wang JB, Lu J, et al. Comprehensive complication index predicts cancer-specific survival of patients with postoperative complications after curative resection of gastric cancer. Gastroenterol Res Pract. 2018; 2018:4396018. PMID:
30581463.

20. Clinical Outcomes of Surgical Therapy Study Group. Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004; 350:2050–2059. PMID:
15141043.

21. Hübner M, Diana M, Zanetti G, Eisenring MC, Demartines N, Troillet N. Surgical site infections in colon surgery: the patient, the procedure, the hospital, and the surgeon. Arch Surg. 2011; 146:1240–1245. PMID:
21768407.
22. Smith RL, Bohl JK, McElearney ST, Friel CM, Barclay MM, Sawyer RG, et al. Wound infection after elective colorectal resection. Ann Surg. 2004; 239:599–607. PMID:
15082963.

23. Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, et al. Postoperative ileus: pathophysiology, incidence, and prevention. J Visc Surg. 2016; 153:439–446. PMID:
27666979.

24. Borchert DH, Federlein M, Müller VA, Wagenpfeil S, Eisele RM. Comprehensive complication index for NOTES procedures: results from a randomized controlled trial and comparison to published NOTES complication data. Surg Endosc. 2015; 29:2928–2933. PMID:
25539692.

25. Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: a review. JAMA Surg. 2017; 152:292–298. PMID:
28097305.
26. Slankamenac K, Nederlof N, Pessaux P, de Jonge J, Wijnhoven BP, Breitenstein S, et al. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014; 260:757–763. PMID:
25379846.
27. Spannenburg L, Sanchez Gonzalez M, Brooks A, Wei S, Li X, Liang X, et al. Surgical outcomes of colonic stents as a bridge to surgery versus emergency surgery for malignant colorectal obstruction: a systematic review and meta-analysis of high quality prospective and randomised controlled trials. Eur J Surg Oncol. 2020; 46:1404–1414. PMID:
32418754.

28. Cheynel N, Cortet M, Lepage C, Benoit L, Faivre J, Bouvier AM. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum. 2007; 50:1568–1575. PMID:
17687610.

29. Yang XF, Pan K. Diagnosis and management of acute complications in patients with colon cancer: bleeding, obstruction, and perforation. Chin J Cancer Res. 2014; 26:331–340. PMID:
25035661.
30. Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. 1994; 81:1270–1276. PMID:
7953385.

31. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–1812. PMID:
12813013.

32. Bray C, Bell LN, Liang H, Haykal R, Kaiksow F, Mazza JJ, et al. Erythrocyte sedimentation rate and c-reactive protein measurements and their relevance in clinical medicine. WMJ. 2016; 115:317–321. PMID:
29094869.
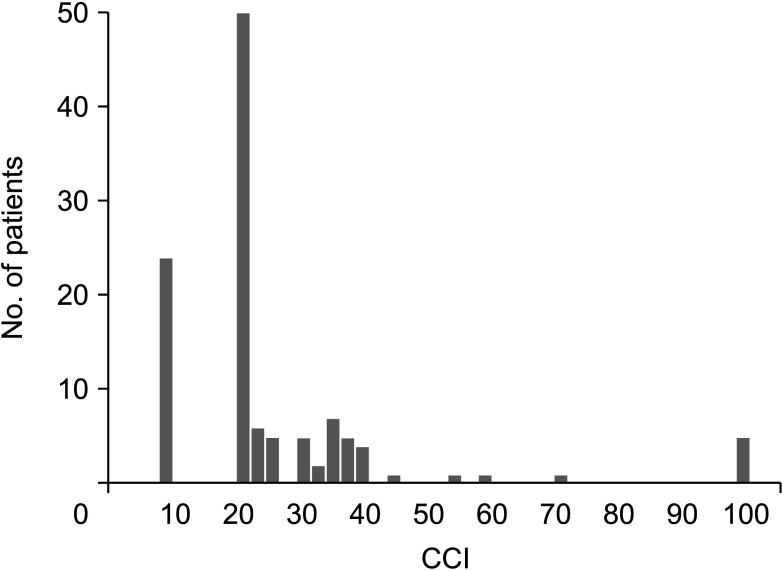
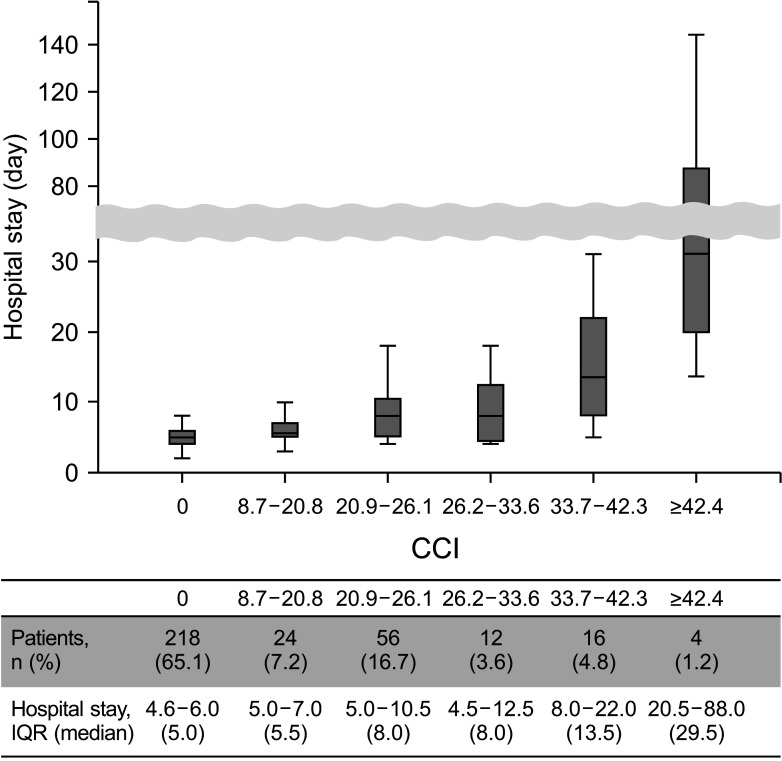
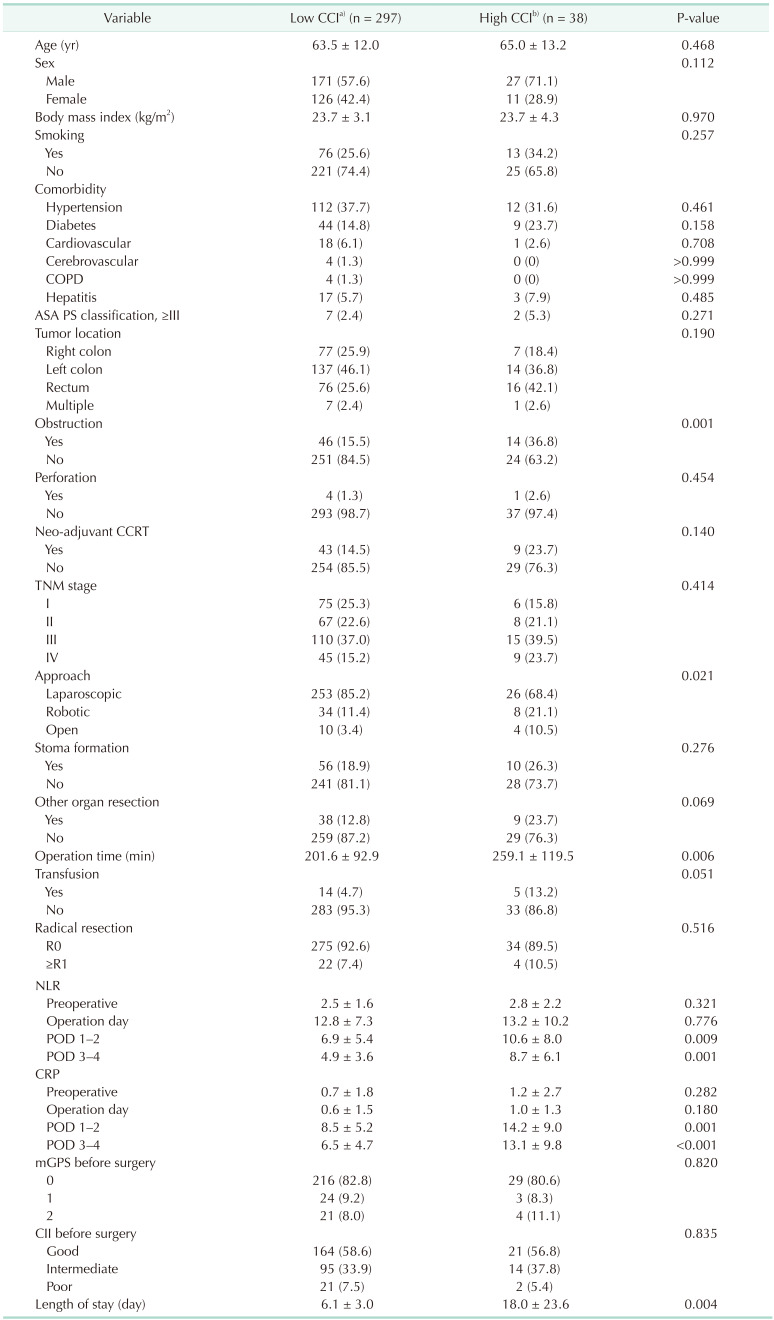
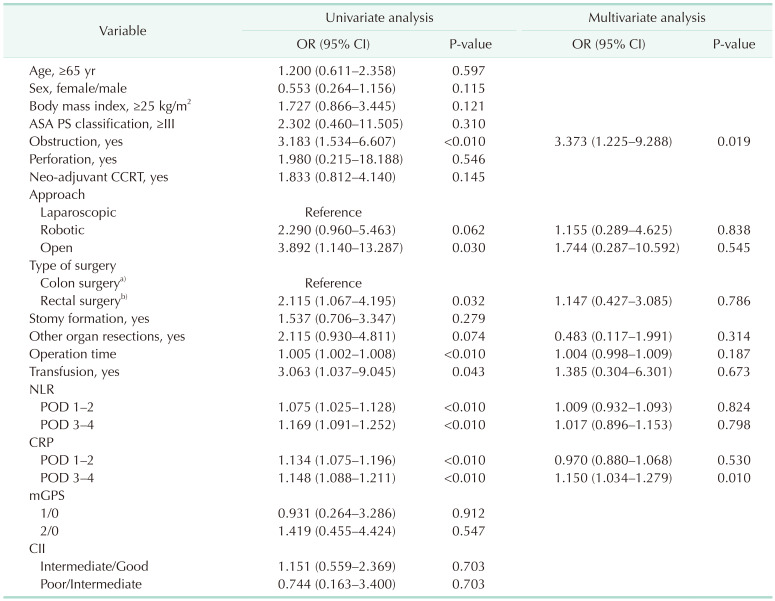
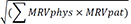 [918]. Also, it can be easily calculated by using the tool provided by the webpage (https://www.assessurgery.com). Severe complications (CDC IIIa or higher) require immediate intervention. Based on previous studies, we defined the cutoff value for high CCI as 26.2. This value corresponds to 1 CDC grade IIIa [19]. The groups were divided into high (CCI ≥ 26.2) and low (CCI < 26.2). The predictive factor analysis for the high CCI group was also performed.
[918]. Also, it can be easily calculated by using the tool provided by the webpage (https://www.assessurgery.com). Severe complications (CDC IIIa or higher) require immediate intervention. Based on previous studies, we defined the cutoff value for high CCI as 26.2. This value corresponds to 1 CDC grade IIIa [19]. The groups were divided into high (CCI ≥ 26.2) and low (CCI < 26.2). The predictive factor analysis for the high CCI group was also performed.



 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download