Abstract
Purpose
The Breast Imaging Reporting and Data System (BI-RADS) is a systematic and standardized scheme of the radiological findings of breast. However, there were different BI-RADS categories between breast cancers as the clinical characteristics in previous studies. We analyzed the association of BI-RADS categories with the clinicopathological characteristics and prognosis of breast cancer.
Methods
A total of 44,184 patients with invasive breast cancers assigned to BI-RADS category 3, 4, or 5 in preoperative mammography or ultrasonography were analyzed retrospectively using large-scale data from the Korean Breast Cancer Society registration system. The difference in the clinicopathological factors and prognoses according to the BI-RADS categories (BI-RADS 3–4 and BI-RADS 5) were compared between the mammography and ultrasonography groups. Comparisons of the clinicopathological factors in both groups were made using logistic regression analysis, while the prognoses were based on the breast cancer-specific survival using the Kaplan-Meier method and Cox proportional hazards model.
Results
The factors associated with BI-RADS were T stage, N stage, palpability, histology grade, and lymphovascular invasion in the mammography group; and N stage, palpability, histology grade, and lymphovascular invasion in the ultrasonography group. In the survival analysis, there were significant differences in the breast cancer-specific survival of the BI-RADS category groups in both of the mammography (hazard ratio [HR], 3.366; P < 0.001) and ultrasonography (HR, 2.877; P < 0.001) groups.
Breast cancer is the commonest cancer in women, both worldwide [12] and in the Republic of Korea [34]. Its incidence is increasing; one of the reasons is the increase in early detection of breast cancer due to the increase in screening using radiological equipment [56].
Mammography is mainly used for breast cancer screening. The Breast Imaging Reporting and Data System (BI-RADS), established by the American College of Radiology, is a systematic and standardized scheme of the radiological findings of breast, which facilitates clear and consistent communication between clinicians and in patient follow-up. BI-RADS was first used in mammography in 1993. Since then, there have been 4 revisions in 1995, 1998, 2003, and 2013. In 2003, BI-RADS was first used in ultrasonography [78].
The currently used BI-RADS classification is the 5th edition, which classifies BI-RADS into 7 categories, from 0 to 6. Among them, BI-RADS categories 1 to 5 reflect the malignancy probability before the diagnosis of breast cancer is made. Based on the malignancy probability: BI-RADS 1 represents a negative (probability of 0%); BI-RADS 2 represents a benign (probability of 0%); BI-RADS 3 represents a probably benign (probability of <2%); BI-RADS 4 represents a suspicious abnormality (probability of 2%–95%); and BI-RADS 5 is highly suggestive of malignancy (probability of ≥95%)—the higher the BI-RADS category, the higher the probability for breast cancer [9].
Although the BI-RADS classification serves as a tool for predicting the probability of breast cancer, there are few domestic studies on whether BI-RADS categories can predict the tumor characteristics and prognosis of patients with breast cancer [10]. Therefore, we investigated whether BI-RADS categories are associated with clinicopathological factors such as clinical characteristics and pathologic findings and breast cancer survival.
In this study, data from the Korean Breast Cancer Society registration system (KBCR) were analyzed. The KBCR is a case registration database involving patients with breast cancer from more than 100 hospitals nationwide since 1996 and has been described in previous studies [41112]. To compile complete death statistics, the causes and dates of death were obtained from the Korea Central Cancer Registry (Ministry of Health and Welfare in collaboration with the Korean National Statistical Office), which was updated in 2014. This study was approved by the Institutional Review Board of Daejeon St. Mary's Hospital (No. DC20ZASI0070) and was conducted in accordance with the tenets of the Declaration of Helsinki. Informed consent from the patients was not required in this study.
Of 115,319 patients registered in the KBCR, our study was a retrospective analysis of 44,184 patients diagnosed invasive breast cancers with prediagnostic malignancy probability in radiological findings such as BI-RADS 3, 4, or 5 between April 1983 and December 2014, using mammography or ultrasonography in the routine preoperative diagnostic process. The clinicopathological factors included age, T stage, N stage, BI-RADS categories of mammography or ultrasonography, menopause, palpability, estrogen receptor (ER) or progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), histologic type, histology grade, nuclear grade, and lymphovascular invasion. The exclusion criteria were as follows: T0, Tis, or unknown T stage; BI-RADS 0, 1, 2, 6, or unknown in both mammography and ultrasonography; and unknown survival status. Depending on which diagnostic imaging method was used, the included patients were classified into the mammography and ultrasonography groups independently. Each group was further divided into 2 groups, BI-RADS 3–4 and 5 groups; clinicopathological features and prognoses were compared between the BI-RADS groups (Fig. 1). Prognoses were compared based on breast cancer-specific survival (BCSS).
Comparisons of the clinicopathological characteristics between the mammography and ultrasonography groups were made using the Student t-test and chi-square test. In addition, the associations between each BI-RADS group and the other clinicopathological factors were compared using logistic regression analysis. The survival rates between the BI-RADS groups were compared using the Kaplan-Meier method and Cox proportional hazards model. All statistical analyses were performed using IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA), and P-values of <0.05 were considered to be statistically significant.
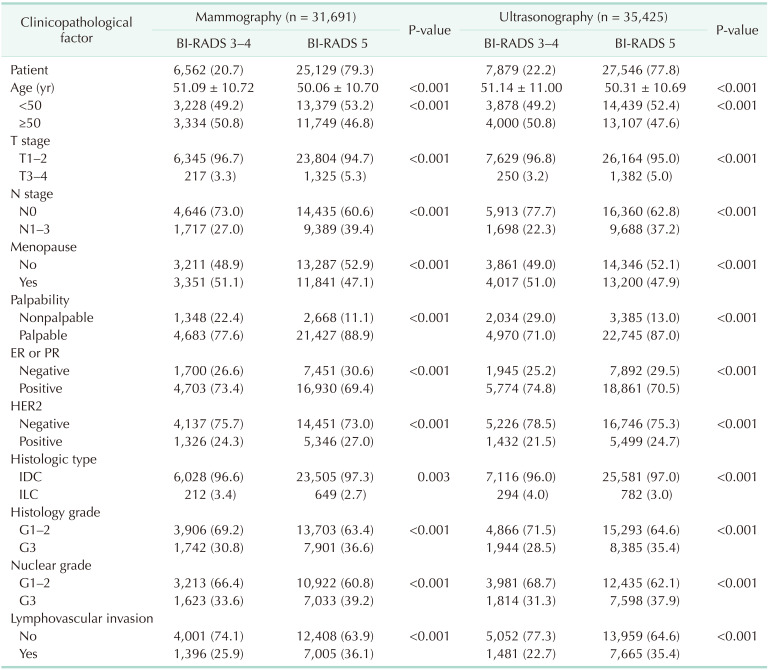
Of the 44,184 patients, there were 31,691 in the mammography group, and 35,425 in the ultrasonography group. Significant differences in all clinicopathological factors between the BI-RADS 3–4 and 5 groups are shown in both the mammography and ultrasonography groups. Compared to the BI-RADS 3–4 group, the BI-RADS 5 group had a greater proportion of patients who were relatively less than 50 years old, in premenopause, had high T and N stages, palpable masses, ER- or PR-negative tumors, HER2-positive tumors, invasive ductal carcinoma, a high histology and nuclear grade, and lymphovascular invasion features (Table 1).
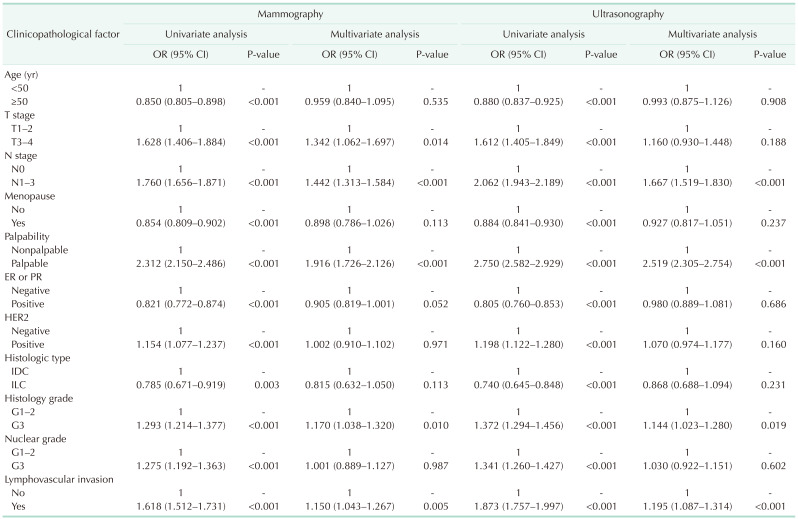
In the univariate analysis, all clinicopathological factors were associated with the BI-RADS groups in both the mammography and ultrasonography groups. In the multivariate analysis, the factors associated with BI-RADS 5 were: T stage (odds ratio [OR], 1.342; P = 0.014), N stage (OR, 1.442; P < 0.001), palpability (OR, 1.916; P < 0.001), histology grade (OR, 1.170; P = 0.010), and lymphovascular invasion (OR, 1.150; P = 0.005) in the mammography group; and N stage (OR, 1.667; P < 0.001), palpability (OR, 2.519; P < 0.001), histology grade (OR, 1.144; P = 0.019), and lymphovascular invasion (OR, 1.195; P < 0.001) in the ultrasonography group (Table 2).
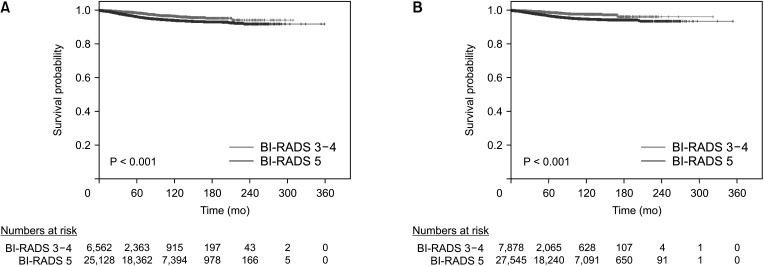
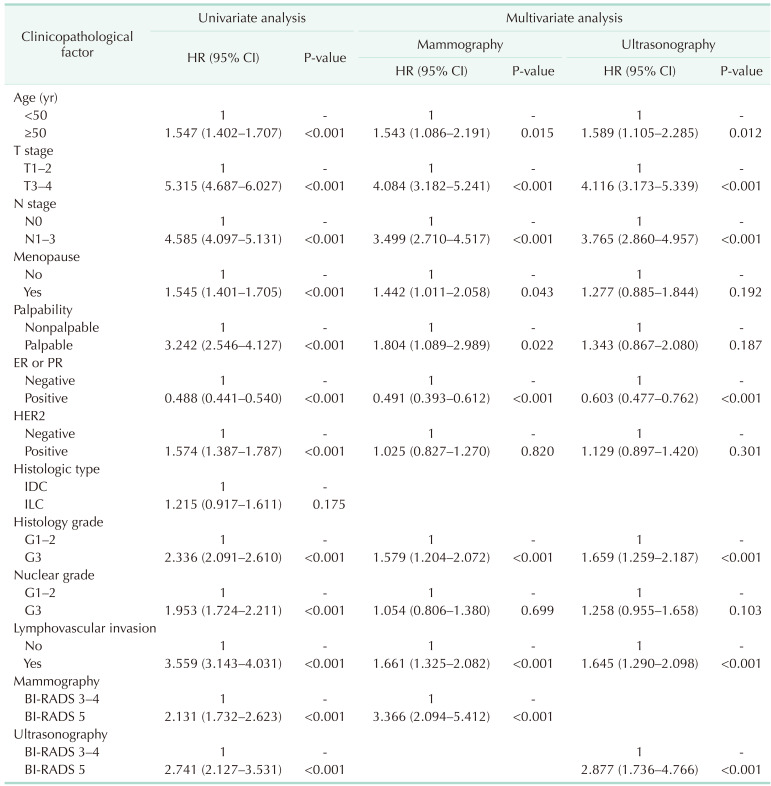
The median follow-up duration was 78 months (range, 0–359 months). Using the Kaplan-Meier method, the 5-year survival rates of the BI-RADS 3–4 and BI-RADS 5 groups were 98.6% and 96.3% (P < 0.001), respectively, in the mammography group; and 98.9% and 96.9% (P < 0.001), respectively, in the ultrasonography group. The 10-year survival rates were 96.6% and 94.1% (P < 0.001), respectively, in the mammography group; and 97.8% and 94.9% (P < 0.001), respectively, in the ultrasonography group (Fig. 2). Using the Cox proportional hazards model, the BCSS of the BI-RADS 5 group was significantly worse than that of the BI-RADS 3–4 group in both of the mammography (hazard ratio [HR], 3.366; P < 0.001) and ultrasonography (HR, 2.877; P < 0.001) groups. Among the factors associated with the BI-RADS categories in the multivariate logistic regression analysis, the factors that affected BCSS were T stage (HR, 4.084; P < 0.001), N stage (HR, 3.499; P < 0.001), palpability (HR, 1.804; P = 0.022), histology grade (HR, 1.579; P < 0.001), and lymphovascular invasion (HR, 1.661; P < 0.001) in the mammography group; and N stage (HR, 3.765; P < 0.001), histology grade (HR, 1.659; P < 0.001), and lymphovascular invasion (HR, 1.645; P < 0.001) in the ultrasonography group (Table 3).
In this study, we found that BI-RADS 5 was associated with more aggressive clinicopathological characteristics than BI-RADS 3–4. In the multivariate analysis, lymph node metastasis, palpable mass, histology grade 3, and lymphovascular invasion were significant factors in the BI-RADS 5 of both mammography and ultrasonography groups.
Several previous studies have reported the associations between the radiological findings and immunohistochemistry of breast cancer [13141516]. However, no such association was found in this study between the BI-RADS categories and immunohistochemistry. Though radiological findings of malignancy are reflected at the molecular level (e.g., immunohistochemistry), changes in the radiological findings seem to have only a slight effect on BI-RADS categories. Ye et al. [17] showed no difference in the molecular subtypes in the different BI-RADS categories. Nuclear grade is part of the Elston and Ellis modification of the Bloom and Richardson system that evaluates histology grade by assessing the nuclear morphology; nuclear grade was not associated with BI-RADS categories in this study. Therefore, nuclear grade is limited to reflecting features of the nuclei of breast cancer cells, and its influence on the BI-RADS categories is small. Histology grade and lymphovascular invasion can identify morphological changes in tissue; they differed with the BI-RADS category in both the mammography and ultrasonography groups and were also associated with prognosis in this study. Several studies have also shown that breast cancer findings vary with the histology grade [131819] or presence of lymphovascular invasion [2021] in mammography or ultrasonography. Blaichman et al. [18] reported that BI-RADS could predict the histology grade. Thus, the BI-RADS categories of mammography and ultrasonography are thought to reflect changes in breast cancer morphologies such as histology grade and lymphovascular invasion.
A palpable mass is a finding on self-examination or physical examination, accounting for the largest proportion of breast cancer symptoms [22]. There are several reports that the palpability of a mass is subjective, but also related to prognosis [232425]. According to this study, a palpable mass is associated with BI-RADS 5, which affects prognosis, and suggests that radiological examinations such as mammography or ultrasonography are necessary if there is a palpable mass on physical examination.
Unlike ultrasonography, mammography has more associated factors, and the difference in prognosis is noticeable; this is thought to be the cause of the difference in the sensitivity of imaging methods. Several studies have shown that mammography has a low sensitivity due to factors such as breast density [2627]. Some of the findings from the ultrasonography may not be visible in the mammography because they are obscured by the breast parenchyma. Thus, as the mammography findings at the boundary of the BI-RADS categories appear to be less frequent than the ultrasonography findings, the gap in the aggressivity between the BI-RADS categories is relatively large, compared to ultrasonography; this is thought to cause the differences in prognosis. Because dense breasts are common among Korean women, the site of the mass seen during mammography tends to be the periphery of the breast parenchyma than the center [28], such as closer to the skin or muscle. Therefore, if a mass is found during mammography, the prognosis is thought to be worse than that of ultrasonography due to the possibility of skin or muscle invasion.
In the mammography and ultrasonography groups in our study, when other factors were adjusted, the prognoses in the BI-RADS 5 groups were worse than in the BI-RADS 3–4 groups. Kim et al. [10] reported that BI-RADS 5 had lower disease-free survival in stage I breast cancer than BI-RADS 3–4 in ultrasonography. In addition, the factors associated with BI-RADS categories were generally associated with prognoses in this study, especially in the mammography group wherein the HR was relatively higher than in the ultrasonography group, and all significant factors in the mammography group were associated with prognoses. This suggests that mammography has a lower sensitivity, and therefore, more active treatment should be considered if a breast tumor with highly suspicious features such as BI-RADS 5 features is found during mammography. Regarding past studies on breast cancer screening, mammographies contribute to increased survival rates [2930], and mammography findings can be considered as predictive factors for prognoses.
This study has several limitations. First, it is a retrospective study. Second, BI-RADS is a system that organizes the degree of suspicious lesions of the breast; however, some findings have been determined using BI-RADS based on the experience and subjectivity of the radiologists. Therefore, similar lesions can have different BI-RADS categories with the possibility of biases. Third, most of the excluded patients had BI-RADS 6, which was as many as those included in the analysis. It was thought to be the result of retrospective data registration. Most of BI-RADS 6 were likely BI-RADS 3, 4, or 5 in real-world radiological findings, which serve as a limitation that failed to include in the analysis. Finally, data on lesion shapes or calcifications for determining the BI-RADS categories in mammography or ultrasonography was lacking, and therefore the classification was not detailed.
However, this study was an objective analysis using large-scale data from multiple hospitals. In addition, while most studies compare the radiological findings and prognosis using only 1 imaging method, this study used 2 imaging methods (mammography and ultrasonography), while identifying the association of the BI-RADS categories with clinicopathological factors and prognosis.
The BI-RADS categories of mammography and ultrasonography are shown to be directly or indirectly related to the prognosis of the patient through the significant associated factors, which can serve as an important factor in making treatment decisions. Furthermore, differences in prognosis among the BI-RADS categories in mammography are higher than those in ultrasonography, which may be due to the relatively low sensitivity of mammography. Further research is required to verify these findings.
References
1. Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019; 144:1941–1953. PMID: 30350310.

2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.

3. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020; 52:335–350. PMID: 32178489.

4. Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020; 23:115–128. PMID: 32395372.

5. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of Korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400. PMID: 23346167.

6. Shin HR, Joubert C, Boniol M, Hery C, Ahn SH, Won YJ, et al. Recent trends and patterns in breast cancer incidence among Eastern and Southeastern Asian women. Cancer Causes Control. 2010; 21:1777–1785. PMID: 20559704.

7. Rao AA, Feneis J, Lalonde C, Ojeda-Fournier H. A pictorial review of changes in the BI-RADS fifth edition. Radiographics. 2016; 36:623–639. PMID: 27082663.

8. Lo JY, Markey MK, Baker JA, Floyd CE Jr. Cross-institutional evaluation of BI-RADS predictive model for mammographic diagnosis of breast cancer. AJR Am J Roentgenol. 2002; 178:457–463. PMID: 11804918.

9. D'Ors CJ. ACR BI-RADS atlas: breast imaging reporting and data system. Reston (VA): American College of Radiology;2013.
10. Kim JY, Jung EJ, Park T, Jeong SH, Jeong CY, Ju YT, et al. Prognostic importance of ultrasound BI-RADS classification in breast cancer patients. Jpn J Clin Oncol. 2015; 45:411–415. PMID: 25670765.

11. Park EH, Min SY, Kim Z, Yoon CS, Jung KW, Nam SJ, et al. Basic facts of breast cancer in Korea in 2014: the 10-year overall survival progress. J Breast Cancer. 2017; 20:1–11. PMID: 28382089.

12. Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016; 19:1–7. PMID: 27066090.

13. Tamaki K, Ishida T, Miyashita M, Amari M, Ohuchi N, Tamaki N, et al. Correlation between mammographic findings and corresponding histopathology: potential predictors for biological characteristics of breast diseases. Cancer Sci. 2011; 102:2179–2185. PMID: 21895869.

14. Xu J, Li F, Chang F. Correlation of the ultrasound imaging of breast cancer and the expression of molecular biological indexes. Pak J Pharm Sci. 2017; 30(4 Suppl):1425–1430. PMID: 29043992.
15. Rashmi S, Kamala S, Murthy SS, Kotha S, Rao YS, Chaudhary KV. Predicting the molecular subtype of breast cancer based on mammography and ultrasound findings. Indian J Radiol Imaging. 2018; 28:354–361. PMID: 30319215.

16. Cen D, Xu L, Li N, Chen Z, Wang L, Zhou S, et al. BI-RADS 3-5 microcalcifications can preoperatively predict breast cancer HER2 and Luminal a molecular subtype. Oncotarget. 2017; 8:13855–13862. PMID: 28099938.

17. Ye DM, Li Q, Yu T, Wang HT, Luo YH, Li WQ. Clinical and epidemiologic factors associated with breast cancer and its subtypes among Northeast Chinese women. Cancer Med. 2019; 8:7431–7445. PMID: 31642614.

18. Blaichman J, Marcus JC, Alsaadi T, El-Khoury M, Meterissian S, Mesurolle B. Sonographic appearance of invasive ductal carcinoma of the breast according to histologic grade. AJR Am J Roentgenol. 2012; 199:W402–W408. PMID: 22915433.

19. Au FW, Ghai S, Lu FI, Moshonov H, Crystal P. Histological grade and immunohistochemical biomarkers of breast cancer: correlation to ultrasound features. J Ultrasound Med. 2017; 36:1883–1894. PMID: 28556296.

20. Liu Z, Li R, Liang K, Chen J, Chen X, Li X, et al. Value of digital mammography in predicting lymphovascular invasion of breast cancer. BMC Cancer. 2020; 20:274. PMID: 32245448.

21. Li JW, Tong YY, Zhou J, Shi ZT, Sun PX, Chang C. Tumor proliferation and invasiveness derived from ultrasound appearances of invasive breast cancers: moving beyond the routine differential diagnosis. J Ultrasound Med. 2020; 39:1589–1599. PMID: 32118315.
22. Mahoney L, Csima A. Efficiency of palpation in clinical detection of breast cancer. Can Med Assoc J. 1982; 127:729–730. PMID: 7139488.
23. Warren SL, Bhutiani N, Agle SC, Martin RC 2nd, McMasters KM, Ajkay N. Differences between palpable and nonpalpable tumors in early-stage, hormone receptor-positive breast cancer. Am J Surg. 2018; 216:326–330. PMID: 29502856.

24. Skinner KA, Silberman H, Sposto R, Silverstein MJ. Palpable breast cancers are inherently different from nonpalpable breast cancers. Ann Surg Oncol. 2001; 8:705–710. PMID: 11597010.

25. Brouckaert O, Schoneveld A, Truyers C, Kellen E, Van Ongeval C, Vergote I, et al. Breast cancer phenotype, nodal status and palpability may be useful in the detection of overdiagnosed screening-detected breast cancers. Ann Oncol. 2013; 24:1847–1852. PMID: 23680691.

26. Rosenberg RD, Hunt WC, Williamson MR, Gilliland FD, Wiest PW, Kelsey CA, et al. Effects of age, breast density, ethnicity, and estrogen replacement therapy on screening mammographic sensitivity and cancer stage at diagnosis: review of 183,134 screening mammograms in Albuquerque, New Mexico. Radiology. 1998; 209:511–518. PMID: 9807581.

27. Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000; 92:1081–1087. PMID: 10880551.

28. Stacey-Clear A, McCarthy KA, Hall DA, Pile-Spellman E, White G, Hulka CA, et al. Mammographically detected breast cancer: location in women under 50 years old. Radiology. 1993; 186:677–680. PMID: 8381550.

29. Autier P, Héry C, Haukka J, Boniol M, Byrnes G. Advanced breast cancer and breast cancer mortality in randomized controlled trials on mammography screening. J Clin Oncol. 2009; 27:5919–5923. PMID: 19884547.

30. Weedon-Fekjær H, Romundstad PR, Vatten LJ. Modern mammography screening and breast cancer mortality: population study. BMJ. 2014; 348:g3701. PMID: 24951459.

Fig. 1
Flow diagram showing the patients selection and categorization process. BI-RADS, Breast Imaging Reporting and Data System.
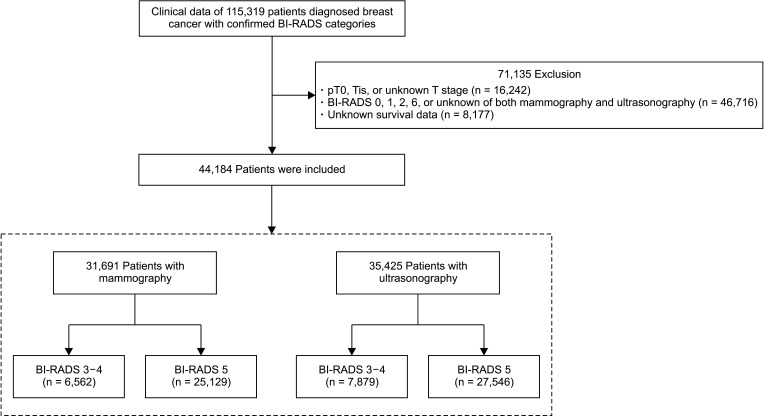
Fig. 2
Breast cancer-specific survival curves of Breast Imaging Reporting and Data System (BI-RADS) 3–4 and 5 groups in the mammography group (A) and ultrasonography group (B).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download