Abstract
Purpose
This study was performed to evaluate the risk of readmission in the first year after low anterior resection (LAR) for patients with rectal cancer and to identify the contributing factors for readmission related to dehydration specifically.
Methods
This was a retrospective analysis of 570 patients who underwent LAR for rectal cancer at National Cancer Center, Republic of Korea. A diverting loop ileostomy was performed in 357 (62.6%) of these patients. Readmission was defined as an unplanned visit to the emergency room or admission to the ward. The reasons for readmission were reviewed and compared between the ileostomy (n = 357) and no-ileostomy (n = 213) groups. The risk factors for readmission and readmission due to dehydration were analyzed using multivariable logistic and Cox proportional hazard model.
Results
Dehydration was the most common cause of readmission in both groups (ileostomy group, 6.7%, and no-ileostomy group, 4.7%, P = 0.323). On multivariable analysis, risk factors for readmission were an estimated intraoperative blood loss of ≥400 mL (odds ratio [OR], 1.757; 95% confidence interval [CI], 1.058–2.918; P = 0.029), and postoperative chemotherapy (OR, 2.914; 95% CI, 1.824–4.653; P < 0.001). On multivariable analysis, postoperative chemotherapy, and not a diverting loop ileostomy, was an independent risk factor for dehydration-related readmission (OR, 5.102; 95% CI, 1.772–14.688; P = 0.003).
Despite recent advances in surgical techniques for low anterior resection (LAR) of rectal cancers, anastomotic leakage remains a major concern after LAR, with a 1.4%–15.2% incidence rate [1234]. In some cases, anastomotic leakage leads to devastating results, such as peritonitis, pelvic abscess, and rectovaginal fistula. To prevent anastomotic leakage, a diverting ileostomy has been commonly used [567]. Ileostomy diverts fecal flow away from the colorectal anastomosis, thus decreasing the risks of severe morbidities associated with anastomotic leakage. The therapeutic benefit of a diverting stoma to decrease the rate of symptomatic anastomotic leakage and reoperation is supported by evidence from a meta-analysis [6].
However, although a diverting ileostomy is used to reduce the rate of complications and is typically reversed within 0.5–1 year after LAR, dehydration, caused by the loss of body fluids, is a complication of a diverting ileostomy and may lead to readmission to manage postoperative dehydration [891011121314151617181920]. Moreover, in some patients, a high-output diverting ileostomy might cause a daily loss of ≥1,200 mL of gastrointestinal (GI) intraluminal fluids, which can significantly lower the volume of body fluid and result in acute renal failure (ARF) due to severe dehydration [10].
Adjuvant chemotherapy, which often causes diarrhea, can worsen dehydration in patients with ileostomy. The cause of readmission related to dehydration after a diverting ileostomy is thus unclear, with patients presenting with combined problems that can cause dehydration, including the ileostomy itself, adjuvant chemotherapy, and large volume of blood loss. If the ileostomy itself was the main cause of dehydration, the ileostomy should be carefully formed, especially for patients at a high risk for dehydration or for ARF. Accordingly, our aim in this study was to evaluate whether a diverting ileostomy itself is the main cause of readmission related to dehydration after LAR for rectal cancer and to identify the risk factors for readmission related to dehydration after LAR.
We retrospectively reviewed the medical records of 570 patients who underwent LAR for rectal cancer at our institution between May 2010 and December 2013. The patients were classified into the ileostomy and no-ileostomy groups, based on the presence or absence of formation of a diverting loop ileostomy, respectively, during LAR. We excluded patients who had a previous history of small bowel resection before surgery and those who underwent small bowel resection concurrently with the LAR, as a short bowel length could increase the output of a diverting ileostomy and increase the risk of dehydration. Patients who required early reversal of the diverting ileostomy due to stoma-related complications during the postoperative period were also excluded.
All study procedures were performed according to the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the National Cancer Center (No. NCC 2019-0018); the need for informed consent was waived owing to the retrospective study design.
The following factors were evaluated as risk factors for readmission related to dehydration after LAR; age, sex, body mass index, American Society of Anesthesiologists physical status classification, comorbidities, previous abdominal surgery history, tumor location and pathologic stage, surgical approach, operative time, blood loss, chemotherapy regimen and radiotherapy, and postoperative length of hospital stay. Comorbidities were classified as renal, cardiovascular, pulmonary, and central nervous system disorders, as well as diabetes mellitus, hypertension, and/or hyperlipidemia.
All diagnoses leading to readmission were thoroughly reviewed retrospectively and categorized as dehydration, ileus/GI obstruction, anal problems, abdominal pain, chemotherapy-induced nausea, stoma problems, genitourinary complications, respiratory complications, surgical site infections, anastomotic leak/strictures, and others. For patients with more than 1 presenting diagnosis, the diagnosis that required admission and/or treatment was considered causative. Patients who underwent readmission for planned ileostomy reversal were excluded.
In this study, rectal cancer was defined as extending up to 15 cm from the anal verge. Readmission was defined as an unplanned visit to the emergency department for observation or intervention, regardless of the length of stay or admission as an inpatient to the hospital. The observation period of readmission was defined between the time of LAR with ileostomy and ileostomy reversal in the ileostomy group and 1 year after surgery in the no-ileostomy group, as 97.1% of ileostomy reversal were performed within 1 year in the ileostomy group.
Dehydration was clinically diagnosed as an ostomy output of ≥1,200 mL within 24 hours in the ileostomy group and a BUN/creatinine level of ≥20 mg/dL and, in both groups, clinical signs of dehydration, such as decreased peripheral perfusion, deep breathing, decreased skin turgor, dry mouth, and increased thirst, and ARF, defined by oliguria, with a production of urine of ≤0.5 mL/kg for ≥6 hours.
The chi-square and Fisher exact tests were used to compare categorical variables between the 2 groups, with a 2-sample t-test or Wilcoxon rank-sum test used to compare continuous variables. All variables with a P-value of <0.1 on univariable logistic regression were entered into a multivariable logistic regression model to estimate the odds ratios (ORs) and associated 95% confidence interval (CIs) for the risk of dehydration. All analyses were conducted with adjustment for all potential confounding factors, using a backward elimination variable selection to identify independent predictive factors for readmission. In addition, a Cox proportional hazard model was used to confirm the factors affecting time to readmission in general and readmission for dehydration specifically. A backward variable selection method with an elimination criterion of 0.05 was used to fit the multivariable Cox proportional model. Inverse probability Kaplan-Meier estimates were used to construct cumulative event plots of readmission and readmission for dehydration. Additionally, a matched analysis, by age and sex, was performed between the ileostomy and no-ileostomy group, using the same follow-up periods for the no-ileostomy group as for the matched patients in the ileostomy group. Statistical significance was defined as P < 0.05 and analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA).
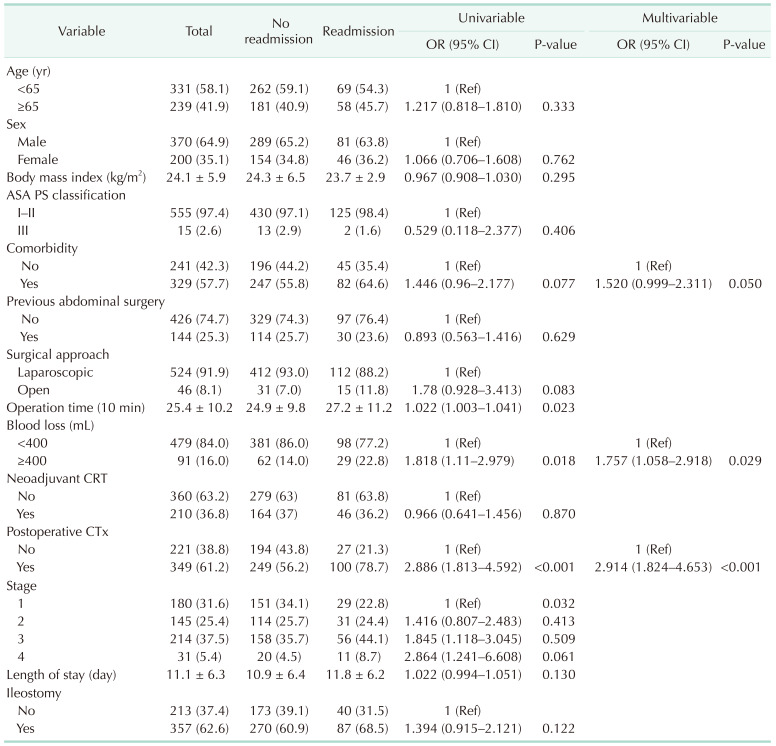
Between 2002 and 2013, 570 patients underwent LAR for rectal cancer at our center. At the time of the primary surgery, a diverting ileostomy was performed in 357 patients (62.6%, the ileostomy group), with the other 213 (37.4%) undergoing LAR without diverting ileostomy (the no-ileostomy group). The demographics of both groups are presented in Table 1. There was no significant difference in the tumor stage between the 2 groups. The ileostomy group presented a longer operative time (P < 0.001), a longer period of hospitalization after LAR (P = 0.012), and a larger volume of blood loss during surgery (P < 0.001). Neoadjuvant chemoradiotherapy was more frequently performed in the ileostomy group (P < 0.001). The postoperative chemotherapy regimen was different between the 2 groups, with the 5-fluorouracil, leucovorin regimen principally used in the ileostomy group and the FOLFOX (5-fluorouracil, leucovorin, oxaliplatin) regimen in the no-ileostomy group (P < 0.001).
The readmission rate was 24.4% in the ileostomy group and 18.8% in the no-ileostomy group (P = 0.121). The rate of readmission during postoperative chemotherapy was similar in both groups (64.2% and 51.5%, respectively; P = 0.224). The median time between discharge and readmission was significantly shorter in the ileostomy than in the no-ileostomy group (25 and 48 days, respectively; P = 0.025), although the length of stay during readmission was similar between the 2 groups (P = 0.942). The mean stoma output at discharge in the ileostomy group was 653 ± 396 mL, with a stoma output of ≥1,200 mL identified in 3.3% of patients.
The most common cause of readmission was dehydration in both groups (6.7% in the ileostomy group and 4.7% in the no-ileostomy group, P = 0.323). The second most common cause of readmission was ileus/GI obstruction (Table 2).
Overall, readmission was observed until 11.8 months after surgery on average (Fig. 1A). On multivariable logistic regression, the risk factors for readmission were as follows (Table 3): a volume of blood loss of ≥400 mL (OR, 1.757; P = 0.029) and administration of postoperative chemotherapy (OR, 2.914; P < 0.001). A diverting ileostomy was not a cause for readmission (P = 0.122) (Table 3, Fig. 2A). Additionally, in the multivariable Cox model, volume of blood loss (hazard ratio [HR], 1.600; P = 0.027) and postoperative chemotherapy (HR, 2.358; P < 0.001) were significant factors for overall readmission (Supplementary Table 1).
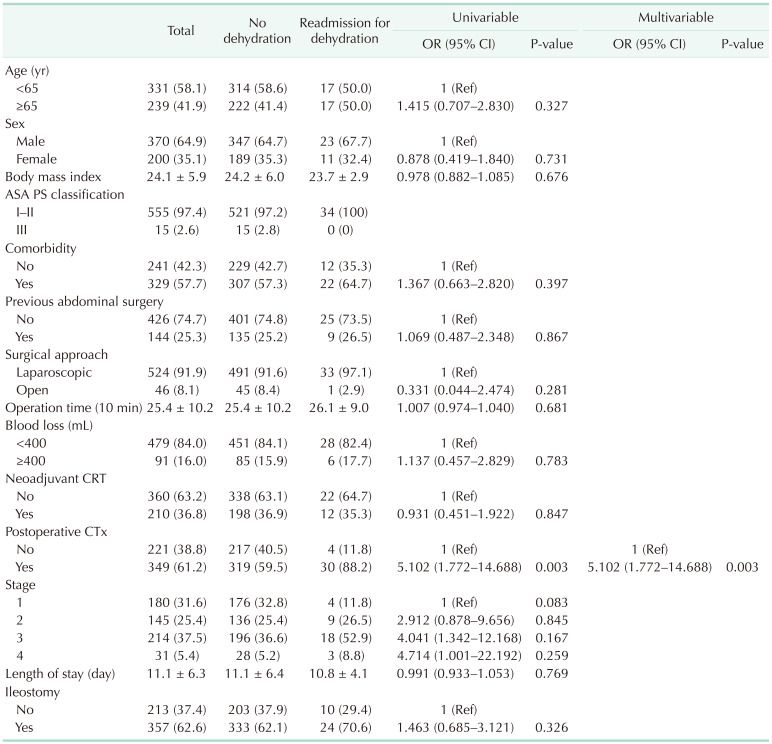
Readmission related to dehydration was observed until 5.7 months after surgery, on average (Fig. 1B). Postoperative chemotherapy was the only factor associated with dehydration-related readmission (OR, 5.102; P = 0.003) (Table 4); again, a diverting ileostomy was not associated with dehydration-related readmission (P = 0.326) (Table 4, Fig. 2B). Only postoperative chemotherapy (HR, 4.702; P = 0.004) was a significant factor in the univariable and multivariable Cox proportional models (Supplementary Table 2).
The patient demographics and clinical characteristics after matching by age and sex are reported in Supplementary Table 3. Even after matching, postoperative chemotherapy remained the only risk factor for overall readmission and readmission related to dehydration (Supplementary Tables 4 and 5).
In this study, we examined the risk factors for readmission and readmission related to dehydration after LAR for rectal cancer. We found that dehydration was the most common cause of readmission in the first year after LAR. However, there was no difference in the overall rates of readmission and readmission specifically related to dehydration among patients with and without a diverting ileostomy after LAR. A volume of blood loss ≥ 400 mL during surgery and postoperative chemotherapy were associated with readmission, with postoperative chemotherapy remaining as the only factor associated with readmission related to dehydration on multivariable analysis.
Although studies have previously evaluated predictors associated with readmission after colorectal surgery, given the inclusion of mixed cohorts, the identified predictors were inconsistent among studies [212223]. Few studies have examined predictors of readmission specifically among patients with rectal cancer. In their population-based comparison of the 30-day readmission rate after both colon and rectal cancer surgery, Doumouras et al. [24] reported that the number of readmissions for renal and stoma-related causes was higher in the rectal cancer group than in the colon cancer group. A recent study identified a high-output stoma as a predictor of readmission following proctectomy for rectal cancer (OR, 11.04; P = 0.003) [25]. Paquette et al. [26] reported age of >50 years as an independent predictor of readmission due to renal failure after ileostomy. Messaris et al. [27] reported postoperative diuretics as the sole risk factor for readmission related to dehydration after ileostomy. Our study included a relatively large number of patients who were readmitted after LAR (127 cases); a diverting ileostomy itself was not related to readmission after LAR, with postoperative chemotherapy being the only independent factor for readmission related to dehydration after LAR.
Previous studies have reported ileostomy to be a risk factor for readmission related to dehydration [2627]. However, only 16%–39% of these study populations comprised patients with rectal cancer, with most of the patients (47%–59%) having inflammatory bowel disease. Moreover, these studies did not clearly state how far the ileostomy was formed proximal to the ileocecal valve. Therefore, it is not possible to differentiate whether dehydration was caused by an underlying disease, a short bowel, or the ileostomy itself. Unlike previous studies, we identified no association between a routine diverting ileostomy combined with LAR and the overall rate of readmission and readmission related to dehydration specifically. We note that our study included only patients who underwent elective LAR for rectal cancer, with the stoma formed approximately 30 cm proximal to the ileocecal valve in all patients in whom a diverting ileostomy was created. This standardization allowed us to evaluate the rate of readmission related to dehydration after LAR by minimizing the effects of other confounding factors.
Unlike most previous studies that examined readmission within 30 or 60 days after discharge, we defined the period of readmission from the time of LAR to reversal of the ileostomy in the ileostomy group and at 1 year after surgery in the no-ileostomy group. The observation period inevitably had to be shorter in the ileostomy group but there were many patients whose period to ileostomy reversal was from 6 months to 1 year after ileostomy (177 of 357, 49.4%). In addition, there were patients in the ileostomy group who did not undergo ileostomy reversal (15 of 357, 4.2%) and, thus, in whom the periods of observation to readmission as up to 1 year after ileostomy. Another important point is that the median time to readmission from discharge was 29 days, with 68% of readmissions occurring within 60 days from discharge and over 70% of patients in the no-ileostomy group being readmitted within 4 months after surgery. Based on these results, we assume that the difference in the period of follow-up between the 2 groups would not have a significant effect on measured outcomes.
To our knowledge, no previous study has reported on the risk of readmission associated with postoperative chemotherapy after LAR for rectal cancer among patients with or without ileostomy. Our finding of postoperative chemotherapy as a risk factor for readmission related to dehydration after LAR for rectal cancer might be attributable to our relatively long period of observation. In our study sample, more than half of the patients in both groups received postoperative chemotherapy. Based on our findings, monitoring for dehydration should be performed in patients scheduled for postoperative chemotherapy. Moreover, sufficient hydration during and after chemotherapy should be performed under close observation.
The limitations of our study need to be acknowledged. First is the retrospective design of the study, with all patients recruited from a single center. Therefore, selection bias could not be eliminated although the data was extracted from the prospectively maintained center database, and patients were excluded according to the predefined criteria. Dehydration was diagnosed from clinical signs and the severity of dehydration was not verified. Therefore, all specific risk factors that might lead to dehydration and subsequent readmission might not have been identified. Operationally, we defined dehydration as an osteotomy output of ≥1,200 mL within 24 hours in the ileostomy group or clinical signs indicative of the need for intervention in both groups. However, the severity of dehydration could not be determined in straight-forwarded fashion and in a large number of patients, the volume of output at the stoma at the time of the readmission was not accurately verified. Finally, because we monitored readmission until the time of stoma reversal, we could not confirm complications and readmission related to stoma closure.
In conclusion, a diverting ileostomy itself after LAR for rectal cancer may not increase the overall rate of readmission and the rate of readmission related to dehydration specifically. In our study sample, the most common cause of readmission after LAR for rectal cancer was dehydration. Postoperative chemotherapy, and not the creation of a diverting ileostomy, was identified as the only risk factor associated with readmission related to dehydration.
Notes
References
1. Mrak K, Jagoditsch M, Eberl T, Klingler A, Tschmelitsch J. Long-term quality of life in pouch patients compared with stoma patients following rectal cancer surgery. Colorectal Dis. 2011; 13:e403–e410. PMID: 21812896.

2. Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD. Postoperative complications following surgery for rectal cancer. Ann Surg. 2010; 251:807–818. PMID: 20395841.

3. Bennis M, Parc Y, Lefevre JH, Chafai N, Attal E, Tiret E. Morbidity risk factors after low anterior resection with total mesorectal excision and coloanal anastomosis: a retrospective series of 483 patients. Ann Surg. 2012; 255:504–510. PMID: 22281734.
4. Kim CH, Lee J, Kwak HD, Lee SY, Ju JK, Kim HR. Tailored treatment of anastomotic leak after rectal cancer surgery according to the presence of a diverting stoma. Ann Surg Treat Res. 2020; 99:171–179. PMID: 32908849.

5. Hallböök O, Sjödahl R. Anastomotic leakage and functional outcome after anterior resection of the rectum. Br J Surg. 1996; 83:60–62. PMID: 8653367.

6. Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009; 96:462–472. PMID: 19358171.

7. Tsikitis VL, Larson DW, Poola VP, Nelson H, Wolff BG, Pemberton JH, et al. Postoperative morbidity with diversion after low anterior resection in the era of neoadjuvant therapy: a single institution experience. J Am Coll Surg. 2009; 209:114–118. PMID: 19651071.

8. Bax TW, McNevin MS. The value of diverting loop ileostomy on the high-risk colon and rectal anastomosis. Am J Surg. 2007; 193:585–588. PMID: 17434360.

9. Mala T, Nesbakken A. Morbidity related to the use of a protective stoma in anterior resection for rectal cancer. Colorectal Dis. 2008; 10:785–788. PMID: 18190612.

10. Thalheimer A, Bueter M, Kortuem M, Thiede A, Meyer D. Morbidity of temporary loop ileostomy in patients with colorectal cancer. Dis Colon Rectum. 2006; 49:1011–1017. PMID: 16598401.

11. Giannakopoulos GF, Veenhof AA, van der Peet DL, Sietses C, Meijerink WJ, Cuesta MA. Morbidity and complications of protective loop ileostomy. Colorectal Dis. 2009; 11:609–612. PMID: 19175642.

12. Chude GG, Rayate NV, Patris V, Koshariya M, Jagad R, Kawamoto J, et al. Defunctioning loop ileostomy with low anterior resection for distal rectal cancer: should we make an ileostomy as a routine procedure?: a prospective randomized study. Hepatogastroenterology. 2008; 55:1562–1567. PMID: 19102343.
13. Chow A, Tilney HS, Paraskeva P, Jeyarajah S, Zacharakis E, Purkayastha S. The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis. 2009; 24:711–723. PMID: 19221766.

14. Mansfield SD, Jensen C, Phair AS, Kelly OT, Kelly SB. Complications of loop ileostomy closure: a retrospective cohort analysis of 123 patients. World J Surg. 2008; 32:2101–2106. PMID: 18563482.

15. Saha AK, Tapping CR, Foley GT, Baker RP, Sagar PM, Burke DA, et al. Morbidity and mortality after closure of loop ileostomy. Colorectal Dis. 2009; 11:866–871. PMID: 19175627.

16. Wong KS, Remzi FH, Gorgun E, Arrigain S, Church JM, Preen M, et al. Loop ileostomy closure after restorative proctocolectomy: outcome in 1,504 patients. Dis Colon Rectum. 2005; 48:243–250. PMID: 15714246.

17. Rathnayake MM, Kumarage SK, Wijesuriya SR, Munasinghe BN, Ariyaratne MH, Deen KI. Complications of loop ileostomy and ileostomy closure and their implications for extended enterostomal therapy: a prospective clinical study. Int J Nurs Stud. 2008; 45:1118–1121. PMID: 18082164.

18. Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. 2010; 12:958–964. PMID: 19604288.

19. Salvadalena G. Incidence of complications of the stoma and peristomal skin among individuals with colostomy, ileostomy, and urostomy: a systematic review. J Wound Ostomy Continence Nurs. 2008; 35:596–607. PMID: 19018200.
20. Fazio VW, Tjandra JJ. Prevention and management of ileostomy complications. J ET Nurs. 1992; 19:48–53. PMID: 1558860.

21. Azimuddin K, Rosen L, Reed JF 3rd, Stasik JJ, Riether RD, Khubchandani IT. Readmissions after colorectal surgery cannot be predicted. Dis Colon Rectum. 2001; 44:942–946. PMID: 11496073.

22. Kariv Y, Wang W, Senagore AJ, Hammel JP, Fazio VW, Delaney CP. Multivariable analysis of factors associated with hospital readmission after intestinal surgery. Am J Surg. 2006; 191:364–371. PMID: 16490548.

23. Snow N, Bergin KT, Horrigan TP. Readmission of patients to the surgical intensive care unit: patient profiles and possibilities for prevention. Crit Care Med. 1985; 13:961–964. PMID: 4053645.
24. Doumouras AG, Tsao MW, Saleh F, Hong D. A population-based comparison of 30-day readmission after surgery for colon and rectal cancer: how are they different? J Surg Oncol. 2016; 114:354–360. PMID: 27334402.

25. O'Connell EP, Healy V, Fitzpatrick F, Higgins CA, Burke JP, McNamara DA. Predictors of readmission following proctectomy for rectal cancer. Dis Colon Rectum. 2019; 62:703–710. PMID: 30762598.
26. Paquette IM, Solan P, Rafferty JF, Ferguson MA, Davis BR. Readmission for dehydration or renal failure after ileostomy creation. Dis Colon Rectum. 2013; 56:974–979. PMID: 23838866.

27. Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, et al. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012; 55:175–180. PMID: 22228161.

SUPPLEMENTARY MATERIALS
Supplementary Tables 1–5 can be found via https://doi.org/10.4174/astr.2021.101.2.111.
Supplementary Table 1
Cox proportional hazard model for time to readmission after low anterior resection
Supplementary Table 2
Cox proportional hazard model for time to readmission related to dehydration after low anterior resection
Supplementary Table 3
Patient demographics and clinical characteristics after matching by age and sex
Supplementary Table 4
Logistic regression of overall readmission and readmission related to dehydration after matching by age and sex
Supplementary Table 5
Cox proportional hazard model of overall readmission and readmission related to dehydration after matching by age and sex
Fig. 1
Cumulative event plot of readmission (A) and readmission related to dehydration (B) after low anterior resection. Overall readmission was observed until 11.8 months after surgery, on average, and readmission related to dehydration specifically until 5.7 months after surgery, on average.
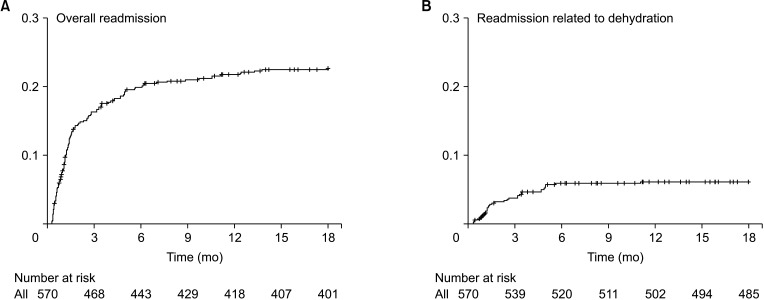
Fig. 2
Cumulative event plot according to ileostomy and readmission (A) and readmission related to dehydration (B) after low anterior resection. Cumulative event plot did not identify an association between presence of an ileostomy and readmission generally and readmission related to dehydration specifically.
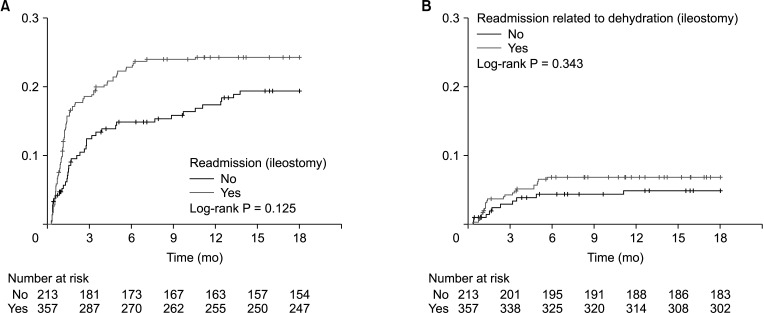
Table 1
Patient demographics and clinical characteristics (n = 570)
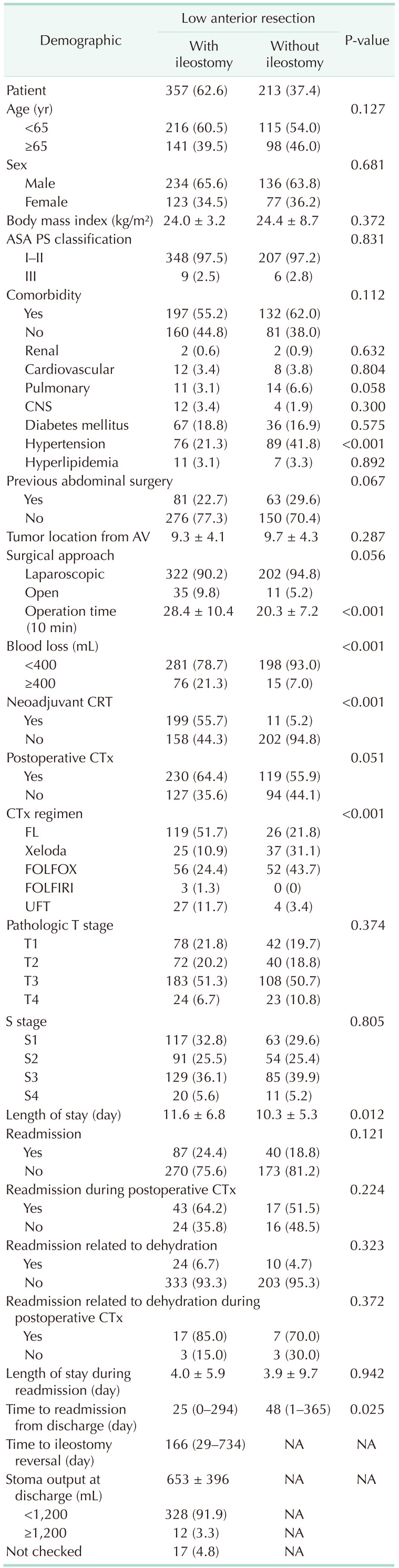
Values are presented as number (%), mean ± standard deviation, or median (range).
ASA, American Society of Anesthesiologists; PS, physical status; AV, anal verge; CRT, chemoradiotherapy; CTx, chemotherapy; FL, 5-fluorouracil/leucovorin; Xeloda, capecitabine; FOLFOX, 5-fluorouracil/leucovorin/oxaliplatin; FOLFIRI, 5-fluorouracil/leucovorin/irinotecan; UFT, uracil/tegafur; NA, not available.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download