Abstract
Purpose
This study aimed to compare treatment options and outcomes based on peritoneal cancer index (PCI) among patients with peritoneal metastasis (PM) of advanced gastric cancer (AGC).
Methods
Between January 2016 and July 2019, clinicopathological data of patients with AGC diagnosed with PM were reviewed. Different treatment methods were performed according to the PCI score: (1) group A (PCI ≤ 13) received cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) with postoperative intraperitoneal (IP) and systemic chemotherapy (n = 29), while (2) group B (PCI > 13) received IP chemotherapy with systemic chemotherapy (n = 22).
Results
Clinical outcomes of 51 patients at the Dankook University Hospital were reviewed. Group A had a significantly lower mean PCI score (9.8 ± 6.9 vs. 32.6 ± 7.1, P < 0.01) than group B, with 25 patients (86.2%) achieving complete cytoreduction. Complications occurred in 16 patients (31.4%), none of who suffered mortality (group A: 11 patients, 37.9% vs. group B: 5 patients, 22.7%; P = 0.25). Among the morbidity, 5 cases (17.2%) and 2 cases (9.1%) exhibited a Clavien-Dindo grade greater than III in groups A and B, respectively (P = 0.04). Groups A and B had an overall median survival time of 34.0 and 16.0 months, respectively (P = 0.03).
Advancements in diagnostic instruments and treatment methods for gastric cancer have continuously progressed. In Korea, gastric cancer treatment outcomes have considerably improved due to the development of endoscopy, technological advancements in surgical instruments including laparoscopy, and the discovery of effective anticancer drugs [123]. Although still remaining as an aggressive and frightening disease with high prevalence, gastric cancer has now been considered as potentially curable. However, this might not be the same for patients with stage IV advanced gastric cancer (AGC), especially those with peritoneal metastasis (PM). Despite constant efforts to control PM of AGC, the prognosis remains poor with a median survival time of 6–14 months [4567]. Most of the patients are diagnosed with “unresectable” disease and receive palliative systemic chemotherapy. Moreover, surgeons have extremely limited options for helping patients with PM of AGC.
At our institution, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been performed since 2013 for patients with PM of AGC, the shortterm outcomes of which have been reported in 2018 [8]. In addition, we have conducted intraperitoneal (IP) chemotherapy since 2017 as a member of Perioperative Intraperitoneal & Systemic Chemotherapy for Gastric Cancer (PIPS-GC) study group. Although our institution is a low-volume center within the province, we have a specialized center with an organized team approach and have much experience with patients suffering from PM. This study aimed to investigate the current strategy and short-term outcomes among patients who underwent surgery for PM of AGC. Moreover, we describe our experience with PM and the role of our specialized center.
Between January 2016 and July 2019, clinicopathological data of patients with AGC diagnosed with PM were reviewed. All diagnoses of PM were confirmed using abdominal CT or laparoscopic examination. The extent of PM was routinely measured using the peritoneal cancer index (PCI) [9]. A cut-off value was set to PCI score of 13, judged in reference to several studies denoting that complete CRS is possible and effective with scores under 13 [810]. Patients were categorized into ‘group A’ with PCI scores of 13 and under, and ‘group B’ with scores exceeding 13. Patients who had distant metastasis, and aged over 70 years were excluded from the study.
The amount of ascites was evaluated using CT and graded as follows (I, none; II, limited to the pelvis; III, beyond the pelvic cavity; and IV, the entire abdomen). Measurable lesions were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [11] at the point of the latest follow-up CT scan (mean follow-up: group A, 18.0 months vs. group B, 11.0 months; P = 0.04). Postoperative morbidity was confined to events occurring within 3 months after the surgery. Severe complications (Clavien-Dindo grade III or greater) were analyzed separately [12].
Our study protocol had been approved by the Institutional Review Board of Dankook University Hospital (No. 2020-04-033), who waived the need for written informed consent. The present study was conducted in accordance with the Declaration of Helsinki.
Different treatment methods were applied according to the patients' PCI score calculated in the operation field. Group A (n = 29) received CRS and HIPEC with IP port insertion. Postoperative IP and systemic chemotherapy were started as soon as the patient was determined to have fully recovered from surgery. Laparoscopic IP port insertion was performed for group B (n = 22) and IP chemotherapy with systemic chemotherapy was carried out 2 weeks postoperatively. The overall treatment pathway is depicted in Fig. 1.
CRS and HIPEC were performed based on the aforementioned standard methods followed at our institution [813]. All grossly visible seeding nodules in parietal peritoneum were removed via peritonectomy, and the nodules in visceral peritoneum were resected along with its organs according to the extent of invasion. Combined resection was performed for colon (18 cases), gallbladder (10 cases), small bowel (7 cases), ovary (7 cases), spleen (5 cases), uterus (3 cases), and pancreas (1 case), each according to the degree of the disease progression. The completeness of the cytoreduction (CCR) score was recorded according to the Sugarbaker classification, with CCR-0 and CCR-1 indicating complete cytoreduction [9]. Mitomycin (30 mg) and cisplatin (90 mg) were used as the chemotherapeutic agents (closed method, 90 minutes, 42℃) in HIPEC. The tip of the IP catheter was stationed in the pouch of Douglas with an anchoring suture in the pelvic cavity to prevent malposition. The subcutaneous tract of the catheter was made as short as possible to avoid kinking. The IP port was placed into the right subcostal area and fixed tightly to the subcutaneous tissue to prevent migration or eversion. Paclitaxel (40 mg/m2 dissolved in 500 mL normal saline over 60 minutes) was selected as the IP chemotherapy agent. The patients received IP chemotherapy based on a 3-week cycle under the condition that there was no severe toxicity or postoperative complications. Various systemic chemotherapies were combined with IP chemotherapy, abided by the guidelines and insurance coverage (Table 1).
Categorical data were assessed using the chi-square test or Fisher exact test, whereas continuous variables were assessed using Student t-test or the Mann-Whitney U-test. Survival outcomes were analyzed using the Kaplan-Meier method and log-rank test. A P-value of <0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics for Windows, ver 22.0 (IBM Corp., Armonk, NY, USA).
A total of 51 patients (27 males and 24 females) with a mean age of 49.5 ± 11.4 years were included. The baseline characteristics of the participants are summarized in Table 1. Synchronous and metachronous PM were identified in 32 patients (62.7%) and 19 patients (37.3%), respectively. The mean number of IP chemotherapy cycles was 6.2 ± 4.0. The main demographic difference between both groups was their American Society of Anesthesiologists physical status classification (P = 0.05), with no other significant differences in patient demographics being observed.
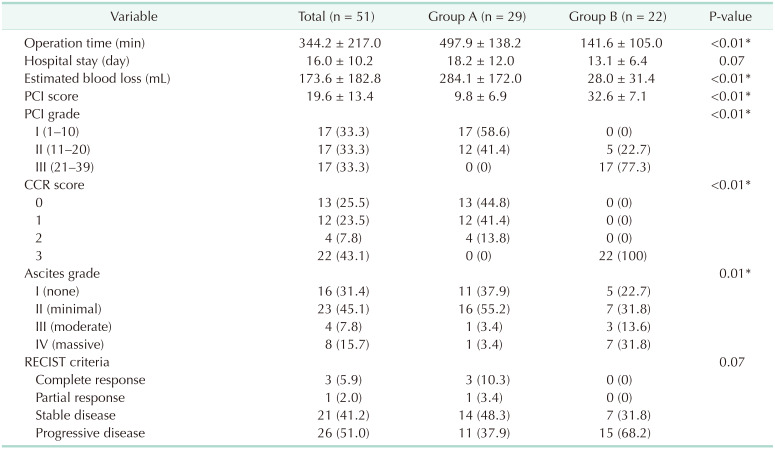
Table 2 shows the surgical results among patients with PM of AGC. Accordingly, group A had significantly longer operation times (497.9 ± 138.2 minutes vs. 141.6 ± 105.0 minutes, P < 0.01) and greater estimated blood loss (284.1 ± 172.0 mL vs. 28.0 ± 31.4 mL, P < 0.01) compared to group B. Moreover, group A had a significantly lower mean PCI score (9.8 ± 6.9 vs. 32.6 ± 7.1, P < 0.01) than group B, with 25 patients (86.2%) achieving complete cytoreduction.
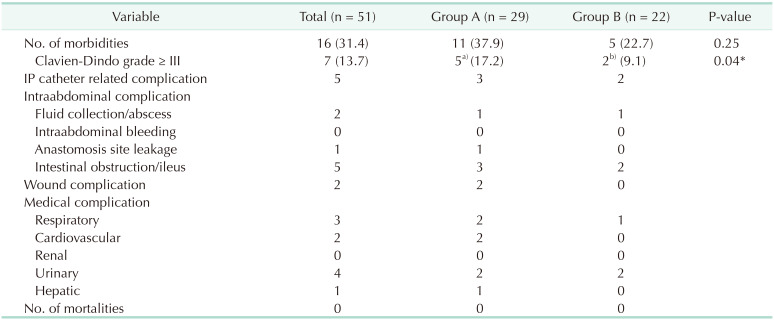
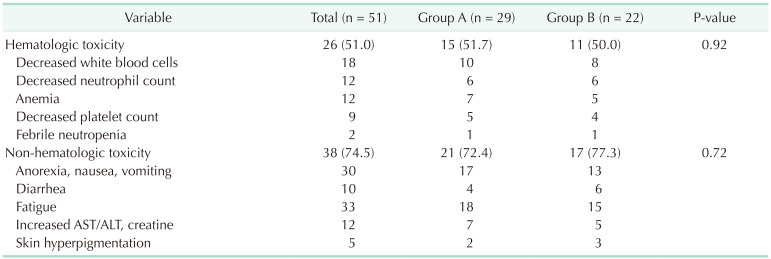
Our results showed a surgical morbidity rate of 31.4% (group A, 37.9% vs. group B, 22.7%; P = 0.25). Among the morbidity, 5 cases (17.2%) and 2 cases (9.1%) exhibited a Clavien-Dindo grade greater than III in groups A and B, respectively (P = 0.04) (Table 3). No mortality had been observed in this study. Moreover, no significant differences in hematologic and non-hematologic adverse events were found between both groups (51.7% vs. 50.0%; P = 0.92 and 72.4% vs. 77.3%; P = 0.72, respectively) (Table 4). Compared to data before 2016, our data showed a higher rate of complete cytoreduction with lower morbidity and mortality following CRS and HIPEC (Fig. 2).
The overall median survival time was 24.0 months (median follow-up, 16.0 months), with a significant difference in survival time being observed between both groups (group A, 34.0 months vs. group B, 16.0 months; P = 0.03) (Fig. 3A). The progression-free median survival period of group A was 23.0 months, and group B was 11.0 months (P = 0.01) (Fig. 3B). Patients who underwent complete cytoreduction had a median survival of 36.0 months, which was significantly longer than the median survival of 17.0 months in patients who did not undergo complete cytoreduction (P = 0.03) (Fig. 4).
Stage IV gastric cancer combined with PM has been a frightening disease given its poor outcomes following both diagnosis and treatment. Comparing to other stage IV states, including distant metastasis, patients with PM experience numerous obstacles related to lesion measurement and assessing responsiveness to treatments [14]. Although several treatment methods have been suggested to control this fatal disease [15], no consensus has yet been achieved with several debates continuing until recently. Guidelines have recommended palliative systemic chemotherapy, which is the only treatment covered by the health insurance system in Korea. Thus, most institutions have followed these guidelines for patients with PM of AGC [1617]. This provides surgeons with limited options compared to medical oncologists who often play an important role in the field of AGC with PM.
Surgeons have continued to search for methods whereby they can actively treat patients with PM and improve prognosis. Our institution has operated as a specialized PM center, offering multimodal treatment concepts that combine systemic chemotherapy, IP chemotherapy, and surgical cytoreduction with HIPEC. CRS has been performed in patients with colorectal cancer and peritoneal pseudomyxoma peritonei since the early 2000s, while HIPEC had first been introduced in 2013 [13]. In addition, we have expanded our criteria for conducting CRS and HIPEC among patients with PM of AGC in 2013 [8] and started IP chemotherapy in 2017. Although our institution is a low-volume center within the province, performing 100–120 gastrectomies annually, we have intensively treated over 25 patients with PM of AGC per year (20%–25% of gastric cancer surgery). Most of these patients had been transferred from high-volume centers in metropolitan areas to seek a second opinion. Distinguished from the high-volume centers which hold many advantages with regards to managing cancer patients, a professionalized low-volume center has its distinct merits in specializing in patients with PM.
Given the importance of continuity and concentration of treatments, all procedures related to PM (surgery, systemic chemotherapy, and IP chemotherapy) in our institution have been performed by surgical oncologists. An interdepartment transfer is not necessary during the management where the patient can receive consistent care, resulting in high compliance. Given that surgical oncologists can provide integrated care starting from initial surgery until postoperative chemotherapy, they are able to follow-up on patients more efficiently [18]. Regarding therapeutic planning, designated surgeons from low-volume centers may be able to provide more comprehensive yet focused treatment to patients with PM compared to those from high-volume centers. Owing to the lack of resident doctors in the department, surgical specialists are compelled to perform almost everything from medical records to the instruction of orders. Moreover, health care providers providing basic care for patients do not rotate every month, and only specialists and professional nurses participate in patient management. Although such system in a low-volume center within the province may certainly seem as a limitation, we have continued to sublimate this situation to our advantage.
During the early stages of our PM center, almost all patients received CRS and HIPEC for AGC with PM regardless of disease severity. However, patients with high PCI scores had a low chance of achieving complete cytoreduction, which resulted in poor survival rates. Moreover, such patients had unacceptable higher postoperative morbidity and mortality compared to those who had undergone major general surgeries [8]. Given that all visible tumors had to be removed to achieve complete cytoreduction, concomitant resection of visceral organs was inevitable, resulting in several serious complications. After joining the PIPS-GC study group, we discussed treatment options with other institutional researchers for patients of PM [19] and ultimately accepted the approach of patient selection based on the PCI score and patients' potential for complete cytoreduction after reflecting on the discussions. We established 2 treatment strategies based on PCI score with a cutoff value of 13 points (Fig. 1). Accordingly, patients with PCI of >13 did not receive CRS and HIPEC but instead received only IP catheterization.
From scrutinizing patient selection and performing CRS with HIPEC only in group A, the outcome of our survival rate was higher compared to other studies [2021]. It can be explained that localized PM with PCI scores below 13 was completely resected by CRS and micrometastasis was managed to be controlled to a certain extent by means of HIPEC followed by IP + systemic chemotherapy. When accompanied with careful patient selection, CRS is a feasible procedure and benefits can be expected in patients with PM of AGC. In group B, it was determined that the extent of PM was severe and only IP chemotherapy was implemented without CRS. In cases of severe PM, the general condition of the patient tends to be poor and it is difficult to continue systemic chemotherapy in many cases, but IP chemotherapy has lower toxicity and shows less invasiveness to the patients [22]. Thereby, it was possible to carry out IP chemotherapy and manage the patients while maintaining a tolerable survival rate.
After experiencing cases of PM, our institution considered some discussion points. First, accurate diagnostic confirmation of PM is a significant aspect to be considered in the treatment of AGC with PM. The importance of patient selection cannot be overstated given that a number of patients with PM will not benefit from CRS and HIPEC due to disease extent [23]. Precise diagnosis is the first step toward enabling efficient patient selection. In patients with metachronous presentations who had already received various types of chemotherapy, determining the exact date of PM remained challenging. Surgical oncologists need to review the medical records meticulously, including radiologic and pathologic findings, to ensure no problems existed during the overall follow-up period. The limitation associated with the scoring system of PM is another factor that hinders treatment strategies. The PCI scoring system has been considered the gold standard for evaluating the extent of disease in most studies regarding PM. However, visual evaluation may provide incorrect estimates of the nodule size and the presence or absence of PM. Recently, Bhatt et al. [24] reported problems with subjectivity in the surgical PCI scoring system. Nonetheless, further studies are needed to objectify the PCI scoring system, such as compulsory reviews through video recording and combination with other grading systems.
Regarding the disease presentation of PM, differences between synchronous and metachronous PM remain unclarified. Most studies on PM involved a combination of both types of disease presentations. However, both disease presentations show clinically distinct patterns during initial detection and disease recurrence. Thus, when planning for CRS and HIPEC among patients with synchronous PM, surgical oncologists need to establish clear imaging-based decisions that are not confounded by the presence of other types of metastases and determine the best first-line chemotherapy. In the case of metachronous PM, unaffected review of the initial surgery is essential, while mechanisms promoting tolerance and resistance to chemotherapy should be reconsidered. Moreover, a molecular biologic approach in understanding PM disease presentation should be utilized to determine prognostic factors.
Finally, continuation of chemotherapy with minimal side effects should be considered as a major factor for improved survival. Since CRS alone is apparently insufficient to improve patient prognosis, IP and systemic chemotherapy should be sustained as long as possible under the condition that it is well tolerated by the patient. IP chemotherapy is advantageous in controlling PM due to its milder systemic toxicity and lower adverse event rates compared to systemic chemotherapy. However, technical problems may occur related to intraabdominal catheter and port used for delivering the chemotherapeutic agents. Several cases of cavitation inside the abdomen have been reported, which resulted in discontinuation of chemotherapy. Future studies should consider these kinds of technical issues in order to improve the continuity of IP chemotherapy. Moreover, systemic chemotherapy should be carried out consecutively before and after the surgery. Patients with PM essentially have poor immunity and nutritional status, which lower their compliance with chemotherapy. Preventing sarcopenia is essential during palliative chemotherapy. Hence, surgical oncologists should collaborate with other departments to provide basic nutritional care for the patients.
Several limitations need to be considered. First, selection bias was inevitable during patient enrollment given that this was a retrospective observational analysis from a single institution collecting data from heterogeneous patients. The heterogeneity of the systemic chemotherapy the patients received can affect their survival rate. Given that phase III studies on PM is difficult to conduct, future studies can be expected to have such a limitation. Second, this study included a very small sample size (n = 51), while long-term results could not be obtained owing to the short follow-up period. Thirdly, it was difficult to compare whether the survival rate was affected by the severity of the disease (the difference in the PCI score) or the difference in the surgical method, because the treatment methods of groups A and B were different. Also, normothermic IP chemotherapy was not established in our institution before 2016, making it impossible for us to compare the survival rate with the previous treatment policy. Lastly, compared to the relatively higher number of complications above Clavien-Dindo III in patients from group A, the number of patients with complete and partial responses was not significantly higher. Three out of 5 morbidities (60.0%) in group A with grades higher than Clavien-Dindo III involved IP catheter occlusion, which is not a grave surgical complication. Efforts, however, must be kept up to reduce the surgical morbidity related to CRS and HIPEC. Further prospective study is essential concerning these issues.
In conclusion, the present study described different treatment methods according to the PCI score among patients with PM of AGC. When accompanied with careful patient selection, our approach may be considered an acceptable option for the treatment of PM of AGC. Additionally, various in-depth studies related to AGC with PM patients are necessary for the future, and we look forward to the day when much attention will be paid to active treatment of PM patients.
References
1. Kim HH, Han SU, Kim MC, Kim W, Lee HJ, Ryu SW, et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: the KLASS-01 randomized clinical trial. JAMA Oncol. 2019; 5:506–513. PMID: 30730546.
2. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820. PMID: 17978289.

3. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.

4. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9:215–221. PMID: 18282805.

5. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–673. PMID: 19153121.

6. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–1235. PMID: 25240821.

7. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–697. PMID: 20728210.

8. Kim DW, Park DG, Song S, Jee YS. Cytoreductive surgery and hyperthermic int raper itoneal chemotherapy as t reatment opt ions for per itoneal metastasis of advanced gastric cancer. J Gastric Cancer. 2018; 18:296–304. PMID: 30276006.
9. Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996; 82:359–374. PMID: 8849962.

10. Rau B, Brandl A, Thuss-Patience P, Bergner F, Raue W, Arnold A, et al. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer. 2019; 22:1226–1237. PMID: 31065877.

11. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.
12. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009; 250:187–196. PMID: 19638912.
13. Jo MH, Suh JW, Yun JS, Namgung H, Park DG. Cytoreductive surgery and intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal cancer: 2-year follow-up results at a single institution in Korea. Ann Surg Treat Res. 2016; 91:157–164. PMID: 27757392.

14. Kodera Y. Surgery with curative intent for stage IV gastric cancer: is it a reality of illusion? Ann Gastroenterol Surg. 2018; 2:339–347. PMID: 30238074.

15. Rizzo A, Mollica V, Ricci AD, Maggio I, Massucci M, Rojas Limpe FL, et al. Third- and later-line treatment in advanced or metastatic gastric cancer: a systematic review and meta-analysis. Future Oncol. 2020; 16:4409–4418. PMID: 31793342.

16. Guideline Committee of the Korean Gastric Cancer Association(KGCA). Development Working Group & Review Panel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19:1–48. PMID: 30944757.
17. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.
18. Min JS, Lee CM, Choi SI, Seo KW, Park DJ, Baik YH, et al. Who can perform adjuvant chemotherapy treatment for gastric cancer? A multicenter retrospective overview of the current status in Korea. J Gastric Cancer. 2018; 18:264–273. PMID: 30276003.

19. Kim DW, Jee YS, Kim CH, Kim JJ, Park S, Choi SI, et al. Multicenter retrospective analysis of intraperitoneal paclitaxel and systemic chemotherapy for advanced gastric cancer with peritoneal metastasis. J Gastric Cancer. 2020; 20:50–59. PMID: 32269844.

20. Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, et al. The 30-year experience: a meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017; 79:1–14. PMID: 28456089.
21. Yarema R, Mielko J, Fetsych T, Ohorchak M, Skorzewska M, Rawicz-Pruszyński K, et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: a retrospective cooperative Central-Eastern European study. Cancer Med. 2019; 8:2877–2885. PMID: 31033239.

22. Chia DK, So JB. Recent advances in intraperitoneal chemotherapy for gastric cancer. J Gastric Cancer. 2020; 20:115–126. PMID: 32595996.

23. Yonemura Y, Prabhu A, Sako S, Ishibashi H, Mizumoto A, Takao N, et al. Long term survival af ter cytoreduct ive surgery combined with perioperative chemotherapy in gastric cancer patients with peritoneal metastasis. Cancers (Basel). 2020; 12:116.
24. Bhatt A, Yonemura Y, Mehta S, Benzerdjeb N, Kammar P, Parikh L, et al. The Pathologic Peritoneal Cancer Index (PCI) strongly differs from the surgical PCI in peritoneal metastases arising from various primary tumors. Ann Surg Oncol. 2020; 27:2985–2996. PMID: 32040698.

Fig. 1
Treatment strategy for advanced gastric cancer (AGC) with peritoneal metastasis (PM) in Dankook University Hospital. PCI, peritoneal cancer index; CRS, cytoreductive surgery; HIPEC, hyperthermic intraperitoneal chemotherapy; IP, intraperitoneal.
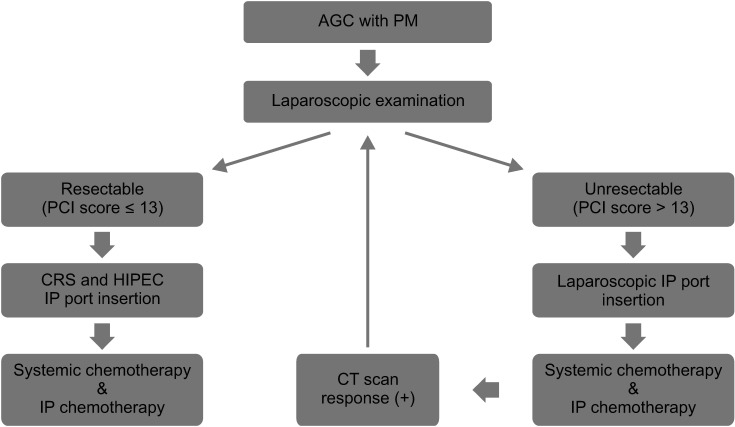
Fig. 2
Comparison of patient information regarding cytoreductive surgery and hyperthermic intraperitoneal chemotherapy before and after 2016. PCI, peritoneal cancer index; CD, Clavien-Dindo.
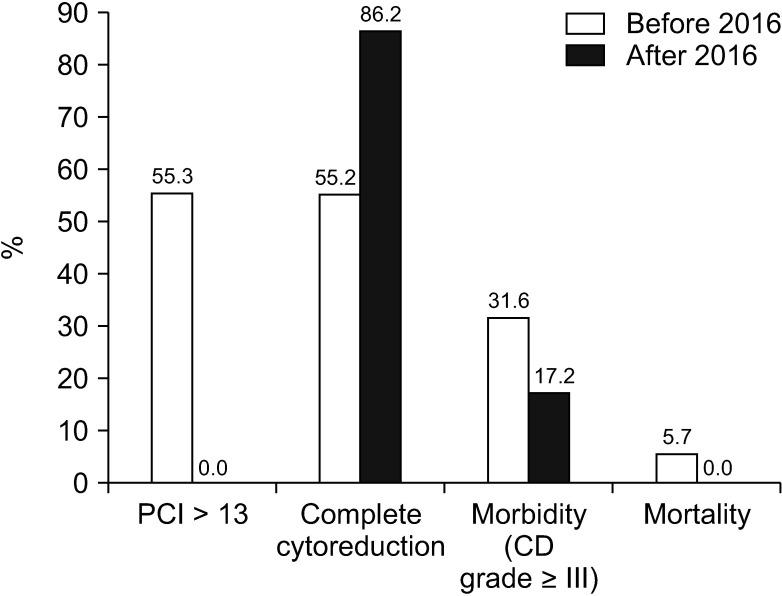
Fig. 3
Overall survival (A) and progression free survival (B) according to treatment type (34.0 months vs. 16.0 months, P = 0.03; 23.0 months vs. 11.0 months, P = 0.01).
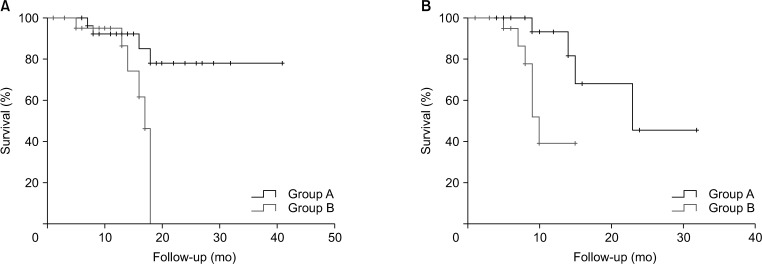
Fig. 4
Overall survival according to the completeness of cytoreduction (CCR) (0–1, 36.0 months and 2–3, 17.0 months; P = 0.03).
Table 1
Patient demographics and baseline characteristics
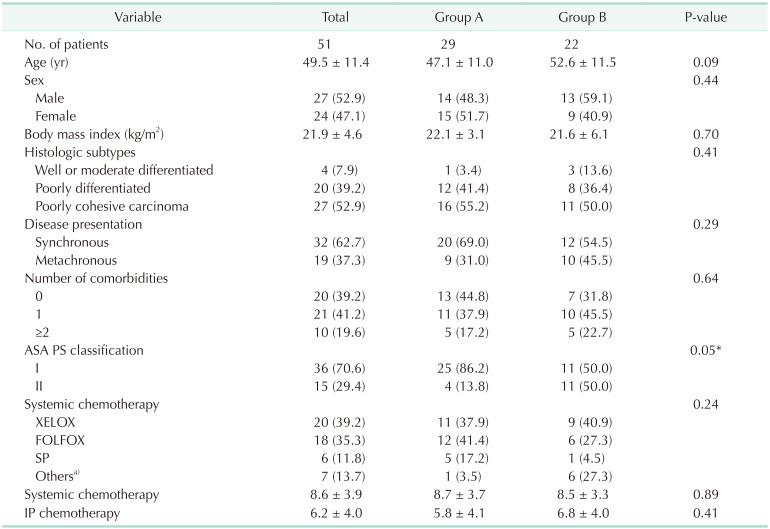
Values are presented as number only, mean ± standard deviation, or number (%).
ASA, American Society of Anesthesiologists; PS, physical status; XELOX, capecitabine and oxaliplatin; FOLFOX, 5-fluorouracil, folinic acid, and oxaliplatin; SP, S-1 (tegafur/gimeracil/oteracil) and cisplatin; IP, intraperitoneal.
a)Weekly paclitaxel, S-1 monotherapy, DFP (docetaxel, 5-fluorouracil, and capecitabine), DCF (docetaxel, cisplatin, and 5-fluorouracil), and FOLFIRI (5-fluorouracil, folinic acid, and irinotecan).
*P < 0.05.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download