1. World Health Organization. Reducing Risks, Promoting Healthy Life. Geneva: WHO;2002.
2. World Health Organization. Adherence to Long-term Therapy: Evidence for Action. Geneva: WHO;2003.
3. Patnode CD, Evans CV, Senger CA, Redmond N, Lin JS. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults without known cardiovascular disease risk factors: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017; 318:175–193. PMID:
28697259.
4. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018; 36:1953–2041. PMID:
30234752.
5. World Health Organization. Macroeconomics and Health: Investing in Health for Economic Development/Report of the Commission on Macroeconomics and Health. Geneva: WHO;2001.
6. Valgimigli M, Garcia-Garcia HM, Vrijens B, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials: a consensus report from the Non-adherence Academic Research Consortium (NARC). Eur Heart J. 2019; 40:2070–2085. PMID:
29992264.

7. Dunbar J. Predictors of patient adherence: patient characteristics. Shumaker SA, Schron EB, Ockene JK, Parker CT, Probstfield JL, Wolle JM, editors. The Handbook of Health Behavior Change. New York (NY): Springer Publishing Company;2004. p. 491–511.
8. Green CA. What can patient health education coordinators learn from ten years of compliance research? Patient Educ Couns. 1987; 10:167–174. PMID:
10285085.

9. Khalil SA, Elzubier AG. Drug compliance among hypertensive patients in Tabuk, Saudi Arabia. J Hypertens. 1997; 15:561–565. PMID:
9170010.

10. Gozzoli V, Palmer AJ, Brandt A, Spinas GA. Economic and clinical impact of alternative disease management strategies for secondary prevention in type 2 diabetes in the Swiss setting. Swiss Med Wkly. 2001; 131:303–310. PMID:
11584692.
11. Morisky DE, Levine DM, Green LW, Shapiro S, Russell RP, Smith CR. Five-year blood pressure control and mortality following health education for hypertensive patients. Am J Public Health. 1983; 73:153–162. PMID:
6849473.

12. Haynes RB, McDonald H, Garg AX, Montague P. Interventions for helping patients to follow prescriptions for medications. Cochrane Database Syst Rev. 2002; CD000011. PMID:
12076376.

13. Ehiri BI. Improving compliance among hypertensive patients: a reflection on the role of patient education. Int J Health Promot Educ. 2000; 38:104–108.

14. Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999; 37:5–14. PMID:
10413387.
15. Rose LE, Kim MT, Dennison CR, Hill MN. The contexts of adherence for African Americans with high blood pressure. J Adv Nurs. 2000; 32:587–594. PMID:
11012800.

16. Horne R. Patients’ beliefs about treatment: the hidden determinant of treatment outcome? J Psychosom Res. 1999; 47:491–495. PMID:
10661596.
17. Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): a new measurement tool for audit and research. Qual Health Care. 2001; 10:135–140. PMID:
11533420.

18. Gupta K, Horne R. The influence of health beliefs on the presentation and consultation outcome in patients with chemical sensitivities. J Psychosom Res. 2001; 50:131–137. PMID:
11316505.

19. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999; 47:555–567. PMID:
10661603.

20. Petrie KJ, Wessely S. Modern worries, new technology, and medicine. BMJ. 2002; 324:690–691. PMID:
11909772.

21. Haisch J, Remmele W. Effectiveness and efficiency of ambulatory diabetes education programs. A comparison of specialty practice and general practice. Dtsch Med Wochenschr. 2000; 125:171–176. PMID:
10719390.
22. Márquez Contreras E, Casado Martínez JJ, Celotti Gómez B, et al. Treatment compliance in arterial hypertension. A 2-year intervention trial through health education. Aten Primaria. 2000; 26:5–10. PMID:
10916893.
23. Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001; CD001481. PMID:
11279717.

24. Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of a medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000; 50:172–175. PMID:
10930970.

25. Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000; 108:20–27. PMID:
11059437.

26. Rice VH, Stead LF. Nursing interventions for smoking cessation. Cochrane Database Syst Rev. 2001; CD001188. PMID:
11686982.

27. Nisbeth O, Klausen K, Andersen LB. Effectiveness of counselling over 1 year on changes in lifestyle and coronary heart disease risk factors. Patient Educ Couns. 2000; 40:121–131. PMID:
10771366.

28. Rohland BM, Rohrer JE, Richards CC. The long-term effect of outpatient commitment on service use. Adm Policy Ment Health. 2000; 27:383–394. PMID:
11077702.
29. Norris SL, Grothaus LC, Buchner DM, Pratt M. Effectiveness of physician-based assessment and counseling for exercise in a staff model HMO. Prev Med. 2000; 30:513–523. PMID:
10901494.

30. Multiple Risk Factor Intervention Trial Research Group. Multiple Risk Factor Intervention Trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. 1982. JAMA. 1997; 277:582–594. PMID:
9032168.
31. Hypertension Detection and Follow-up Program Cooperative Group. Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. 1979. JAMA. 1997; 277:157–166. PMID:
8990344.
32. Belgrave FZ, Lewis DM. The role of social support in compliance and other health behaviors for African Americans with chronic illnesses. J Health Soc Policy. 1994; 5:55–68. PMID:
10138763.

33. Fishman T. The 90-Second Intervention: a patient compliance mediated technique to improve and control hypertension. Public Health Rep. 1995; 110:173–178. PMID:
7630994.
34. Garay-Sevilla ME, Nava LE, Malacara JM, et al. Adherence to treatment and social support in patients with non-insulin dependent diabetes mellitus. J Diabetes Complications. 1995; 9:81–86. PMID:
7599352.

35. Sherbourne CD, Hays RD, Ordway L, DiMatteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: results from the Medical Outcomes Study. J Behav Med. 1992; 15:447–468. PMID:
1447757.

36. Stanton AL. Determinants of adherence to medical regimens by hypertensive patients. J Behav Med. 1987; 10:377–394. PMID:
3669072.

37. Wang CY, Fenske MM. Self-care of adults with non-insulin-dependent diabetes mellitus: influence of family and friends. Diabetes Educ. 1996; 22:465–470. PMID:
8936125.

38. Canga N, De Irala J, Vara E, Duaso MJ, Ferrer A, Martínez-González MA. Intervention study for smoking cessation in diabetic patients: a randomized controlled trial in both clinical and primary care settings. Diabetes Care. 2000; 23:1455–1460. PMID:
11023136.

39. Ockene IS, Hebert JR, Ockene JK, Merriam PA, Hurley TG, Saperia GM. Effect of training and a structured office practice on physician-delivered nutrition counseling: the Worcester-Area Trial for Counseling in Hyperlipidemia (WATCH). Am J Prev Med. 1996; 12:252–258. PMID:
8874688.

40. Ockene IS, Hebert JR, Ockene JK, et al. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH). Arch Intern Med. 1999; 159:725–731. PMID:
10218753.
41. Ockene JK, Adams A, Pbert L, et al. The Physician-Delivered Smoking Intervention Project: factors that determine how much the physician intervenes with smokers. J Gen Intern Med. 1994; 9:379–384. PMID:
7931747.
42. Nguyen TM, La Caze A, Cottrell N. Validated adherence scales used in a measurement-guided medication management approach to target and tailor a medication adherence intervention: a randomised controlled trial. BMJ Open. 2016; 6:e013375.

43. Conn VS, Ruppar TM, Enriquez M, Cooper PS. Patient-centered outcomes of medication adherence interventions: systematic review and meta-analysis. Value Health. 2016; 19:277–285. PMID:
27021763.

44. Algabbani FM, Algabbani AM. Treatment adherence among patients with hypertension: findings from a cross-sectional study. Clin Hypertens. 2020; 26:18. PMID:
32944283.

45. Wei YJ, Simoni-Wastila L, Albrecht JS, et al. The association of antidepressant treatment with COPD maintenance medication use and adherence in a comorbid Medicare population: a longitudinal cohort study. Int J Geriatr Psychiatry. 2018; 33:e212–e220. PMID:
28833488.

46. Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019; 124:1124–1140. PMID:
30920917.

47. Shim JS, Heo JE, Kim HC. Factors associated with dietary adherence to the guidelines for prevention and treatment of hypertension among Korean adults with and without hypertension. Clin Hypertens. 2020; 26:5. PMID:
32190348.

48. Burke LE. Strategies to enhance compliance to weight-loss treatment. Fletcher GF, Grundy SM, Hayman LL, editors. Obesity: Impact on Cardiovascular Disease. Armonk (NY): Futura Publishing Co., Inc;1999. p. 327–343.
49. Prochaska JJ, Zabinski MF, Calfas KJ, Sallis JF, Patrick K. PACE+: interactive communication technology for behavior change in clinical settings. Am J Prev Med. 2000; 19:127–131. PMID:
10913904.

50. Kretchy IA, Owusu-Daaku FT, Danquah SA, Asampong E. A psychosocial perspective of medication side effects, experiences, coping approaches and implications for adherence in hypertension management. Clin Hypertens. 2015; 21:19. PMID:
26893929.

51. Gebreyohannes EA, Bhagavathula AS, Abebe TB, Tefera YG, Abegaz TM. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin Hypertens. 2019; 25:1. PMID:
30675379.

52. Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med. 1997; 19:239–263. PMID:
9603699.

53. Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Medication adherence and use of generic drug therapies. Am J Manag Care. 2009; 15:450–456. PMID:
19589012.
54. Vinker S, Shani M, Baevsky T, Elhayany A. Adherence with statins over 8 years in a usual care setting. Am J Manag Care. 2008; 14:388–392. PMID:
18554077.
55. Parthan A, Vincze G, Morisky DE, Khan ZM. Strategies to improve adherence with medications in chronic, ‘silent’ diseases representing high cardiovascular risk. Expert Rev Pharmacoecon Outcomes Res. 2006; 6:325–336. PMID:
20528525.

56. Belcher DW. Implementing preventive services. Success and failure in an outpatient trial. Arch Intern Med. 1990; 150:2533–2541. PMID:
2244769.

57. Ryan D, Carr A. A study of the differential effects of Tomm’s questioning styles on therapeutic alliance. Fam Process. 2001; 40:67–77. PMID:
11288371.

58. Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009; 75:185–191. PMID:
19013740.

59. Miller W, Rollnick S. Motivational Interviewing. New York (NY): Guilford Press;1999.
60. World Health Organization. Health Promotion Glossary. Geneva: WHO;1998.
61. Webb DG, Horne R, Pinching AJ. Treatment-related empowerment: preliminary evaluation of a new measure in patients with advanced HIV disease. Int J STD AIDS. 2001; 12:103–107. PMID:
11236098.
62. Arnold MS, Butler PM, Anderson RM, Funnell MM, Feste C. Guidelines for facilitating a patient empowerment program. Diabetes Educ. 1995; 21:308–312. PMID:
7621733.

63. Gibson PG, Powell H, Coughlan J, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev. 2002; CD001005. PMID:
12076400.

64. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005; 55:305–312. PMID:
15826439.
65. Shin J, Chia YC, Heo R, et al. Current status of adherence interventions in hypertension management in Asian countries: a report from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2021; 23:584–594.

66. Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983; 51:390–395. PMID:
6863699.

67. Prochaska JO, DiClemente CC. Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992; 28:183–218. PMID:
1620663.
68. de Freitas PP, de Menezes MC, Dos Santos LC, Pimenta AM, Ferreira AV, Lopes AC. The transtheoretical model is an effective weight management intervention: a randomized controlled trial. BMC Public Health. 2020; 20:652. PMID:
32393214.

69. Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011; 171:814–822. PMID:
21555659.

70. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009; 15:e22–e33. PMID:
19514806.
71. Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Curr Med Res Opin. 2012; 28:669–680. PMID:
22429067.

72. Iskedjian M, Einarson TR, MacKeigan LD, et al. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from a meta-analysis. Clin Ther. 2002; 24:302–316. PMID:
11911560.

73. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007; 120:713–719. PMID:
17679131.

74. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed-dose combinations of antihypertensive agents: a meta-analysis. Hypertension. 2010; 55:399–407. PMID:
20026768.
75. Lee HY, Shin J, Kim GH, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019; 25:20. PMID:
31388453.

76. Sherrill B, Halpern M, Khan S, Zhang J, Panjabi S. Single-pill vs free-equivalent combination therapies for hypertension: a meta-analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011; 13:898–909. PMID:
22142349.

77. Kim SJ, Kwon OD, Cho B, Oh SW, Lee CM, Choi HC. Effects of combination drugs on antihypertensive medication adherence in a real-world setting: a Korean Nationwide Study. BMJ Open. 2019; 9:e029862.

78. Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster-randomised trial. Lancet. 2019; 394:672–683. PMID:
31448738.

79. Selak V, Webster R, Stepien S, et al. Reaching cardiovascular prevention guideline targets with a polypill-based approach: a meta-analysis of randomised clinical trials. Heart. 2019; 105:42–48. PMID:
29954855.

80. Muñoz D, Uzoije P, Reynolds C, et al. Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med. 2019; 381:1114–1123. PMID:
31532959.

81. Roy A, Naik N, Srinath Reddy K. Strengths and limitations of using the polypill in cardiovascular prevention. Curr Cardiol Rep. 2017; 19:45. PMID:
28425033.

82. Ockene IS, Hayman LL, Pasternak RC, Schron E, Dunbar-Jacob J. Task force #4--adherence issues and behavior changes: achieving a long-term solution. 33rd Bethesda Conference. J Am Coll Cardiol. 2002; 40:630–640. PMID:
12204492.
83. Jeejeebhoy K, Dhaliwal R, Heyland DK, et al. Family physician-led, team-based, lifestyle intervention in patients with metabolic syndrome: results of a multicentre feasibility project. CMAJ Open. 2017; 5:E229–E236.

84. Rudd P, Miller NH, Kaufman J, et al. Nurse management for hypertension. A systems approach. Am J Hypertens. 2004; 17:921–927. PMID:
15485755.
85. Santschi V, Chiolero A, Burnand B, Colosimo AL, Paradis G. Impact of pharmacist care in the management of cardiovascular disease risk factors: a systematic review and meta-analysis of randomized trials. Arch Intern Med. 2011; 171:1441–1453. PMID:
21911628.
86. Van Zuilen AD, Wetzels JF, Bots ML, Van Blankestijn PJ. MASTERPLAN Study Group. MASTERPLAN: study of the role of nurse practitioners in a multifactorial intervention to reduce cardiovascular risk in chronic kidney disease patients. J Nephrol. 2008; 21:261–267. PMID:
18587712.
87. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. J Am Heart Assoc. 2014; 3:e000718. PMID:
24721801.

88. Janssen V, De Gucht V, Dusseldorp E, Maes S. Lifestyle modification programmes for patients with coronary heart disease: a systematic review and meta-analysis of randomized controlled trials. Eur J Prev Cardiol. 2013; 20:620–640. PMID:
23022703.

89. Bandura A, Schunk DH. Cultivating competence, self-efficacy, and intrinsic interest through proximal self-motivation. J Pers Soc Psychol. 1981; 41:586–598.

90. Becker MH. The Health belief model and personal health behavior. Health Educ Monogr. 1974; 2:324–508.
91. Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychotherapy (Chic). 1982; 19:276–288.

92. Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992; 111:455–474. PMID:
1594721.

93. Fisher JD, Fisher WA, Misovich SJ, Kimble DL, Malloy TE. Changing AIDS risk behavior: effects of an intervention emphasizing AIDS risk reduction information, motivation, and behavioral skills in a college student population. Health Psychol. 1996; 15:114–123. PMID:
8681919.

94. Nolan RP, Upshur RE, Lynn H, et al. Therapeutic benefit of preventive telehealth counseling in the Community Outreach Heart Health and Risk Reduction Trial. Am J Cardiol. 2011; 107:690–696. PMID:
21215382.

95. Friedberg JP, Rodriguez MA, Watsula ME, et al. Effectiveness of a tailored behavioral intervention to improve hypertension control: primary outcomes of a randomized controlled trial. Hypertension. 2015; 65:440–446. PMID:
25403606.
96. Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010; CD005182. PMID:
20238338.

97. Linden W, Moseley JV. The efficacy of behavioral treatments for hypertension. Appl Psychophysiol Biofeedback. 2006; 31:51–63. PMID:
16565886.

98. Shapiro D, Hui KK, Oakley ME, Pasic J, Jamner LD. Reduction in drug requirements for hypertension by means of a cognitive-behavioral intervention. Am J Hypertens. 1997; 10:9–17. PMID:
9008243.

99. Nolan RP, Feldman R, Dawes M, et al. Randomized controlled trial of e-counseling for hypertension: REACH. Circ Cardiovasc Qual Outcomes. 2018; 11:e004420. PMID:
30006474.

100. Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013; 2013:CD000165.

101. Nessman DG, Carnahan JE, Nugent CA. Increasing compliance. Patient-operated hypertension groups. Arch Intern Med. 1980; 140:1427–1430. PMID:
7436639.

102. Yap AF, Thirumoorthy T, Kwan YH. Systematic review of the barriers affecting medication adherence in older adults. Geriatr Gerontol Int. 2016; 16:1093–1101. PMID:
26482548.

103. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005; 353:487–497. PMID:
16079372.

104. Gold N, Durlik C, Sanders JG, Thompson K, Chadborn T. Applying behavioural science to increase uptake of the NHS Health Check: a randomised controlled trial of gain- and loss-framed messaging in the national patient information leaflet. BMC Public Health. 2019; 19:1519. PMID:
31727030.

105. Bittman B, Poornima I, Smith MA, Heidel RE. Gospel music: a catalyst for retention, engagement, and positive health outcomes for African Americans in a cardiovascular prevention and treatment program. Adv Mind Body Med. 2020; 34:8–16. PMID:
32277749.
106. Kim HS, Oh JA. Adherence to diabetes control recommendations: impact of nurse telephone calls. J Adv Nurs. 2003; 44:256–261. PMID:
14641395.

107. Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Jpn J Nurs Sci. 2010; 7:121–128. PMID:
21092015.

108. Agarwal R, Bills JE, Hecht TJ, Light RP. Role of home blood pressure monitoring in overcoming therapeutic inertia and improving hypertension control: a systematic review and meta-analysis. Hypertension. 2011; 57:29–38. PMID:
21115879.
109. Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004; 329:145. PMID:
15194600.

110. Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Am J Hypertens. 1996; 9:285–292. PMID:
8722429.

111. Park E, Kim J. The impact of a nurse-led home visitation program on hypertension self-management among older community-dwelling Koreans. Public Health Nurs. 2016; 33:42–52. PMID:
26250719.

112. VanEpps EM, Troxel AB, Villamil E, et al. Financial incentives for chronic disease management: results and limitations of 2 randomized clinical trials with New York Medicaid patients. Am J Health Promot. 2018; 32:1537–1543. PMID:
29390862.

113. VanEpps EM, Troxel AB, Villamil E, et al. Effect of process- and outcome-based financial incentives on weight loss among prediabetic New York Medicaid patients: a randomized clinical trial. Am J Health Promot. 2019; 33:372–380. PMID:
30021451.

114. Fernandes R, Chinn CC, Li D, et al. A randomized controlled trial of financial incentives for Medicaid beneficiaries with diabetes. Perm J. 2018; 22:17-080.

115. Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012; 156:416–424. PMID:
22431674.
116. Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive-based approaches for weight loss: a randomized trial. JAMA. 2008; 300:2631–2637. PMID:
19066383.
117. Garza KB, Owensby JK, Braxton Lloyd K, Wood EA, Hansen RA. Pilot study to test the effectiveness of different financial incentives to improve medication adherence. Ann Pharmacother. 2016; 50:32–38. PMID:
26447193.

118. Asch DA, Troxel AB, Stewart WF, et al. Effect of financial incentives to physicians, patients, or both on lipid levels: a randomized clinical trial. JAMA. 2015; 314:1926–1935. PMID:
26547464.
119. Kullgren JT, Troxel AB, Loewenstein G, et al. Individual- versus group-based financial incentives for weight loss: a randomized, controlled trial. Ann Intern Med. 2013; 158:505–514. PMID:
23546562.
120. Patel MS, Asch DA, Rosin R, et al. Framing financial incentives to increase physical activity among overweight and obese adults: a randomized, controlled trial. Ann Intern Med. 2016; 164:385–394. PMID:
26881417.
121. Conn VS, Valentine JC, Cooper HM. Interventions to increase physical activity among aging adults: a meta-analysis. Ann Behav Med. 2002; 24:190–200. PMID:
12173676.

122. Tinker LF, Rosal MC, Young AF, et al. Predictors of dietary change and maintenance in the Women’s Health Initiative Dietary Modification Trial. J Am Diet Assoc. 2007; 107:1155–1166. PMID:
17604744.

123. Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010; 122:406–441. PMID:
20625115.
124. Iwahori T, Ueshima H, Ohgami N, et al. Effectiveness of a self-monitoring device for urinary sodium-to-potassium ratio on dietary improvement in free-living adults: a randomized controlled trial. J Epidemiol. 2018; 28:41–47. PMID:
29093302.

125. Fletcher BR, Hartmann-Boyce J, Hinton L, McManus RJ. The effect of self-monitoring of blood pressure on medication adherence and lifestyle factors: a systematic review and meta-analysis. Am J Hypertens. 2015; 28:1209–1221. PMID:
25725092.

126. Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich). 2006; 8:174–180. PMID:
16522994.

127. Stergiou GS, Bliziotis IA. Home blood pressure monitoring in the diagnosis and treatment of hypertension: a systematic review. Am J Hypertens. 2011; 24:123–134. PMID:
20940712.

128. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA. 2014; 312:799–808. PMID:
25157723.
129. Health literacy: report of the Council on Scientific Affairs. Ad Hoc Committee on Health Literacy for the Council on Scientific Affairs, American Medical Association. JAMA. 1999; 281:552–557. PMID:
10022112.
130. Williams MV, Davis T, Parker RM, Weiss BD. The role of health literacy in patient-physician communication. Fam Med. 2002; 34:383–389. PMID:
12038721.
131. Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011; 155:97–107. PMID:
21768583.

132. Howard DH, Gazmararian J, Parker RM. The impact of low health literacy on the medical costs of Medicare managed care enrollees. Am J Med. 2005; 118:371–377. PMID:
15808134.

133. Brega AG, Ang A, Vega W, et al. Mechanisms underlying the relationship between health literacy and glycemic control in American Indians and Alaska Natives. Patient Educ Couns. 2012; 88:61–68. PMID:
22497973.

134. Magnani JW, Mujahid MS, Aronow HD, et al. Health literacy and cardiovascular disease: fundamental relevance to primary and secondary prevention: a scientific statement from the American Heart Association. Circulation. 2018; 138:e48–e74. PMID:
29866648.

135. Safeer RS, Keenan J. Health literacy: the gap between physicians and patients. Am Fam Physician. 2005; 72:463–468. PMID:
16100861.
136. Ferguson MO, Long JA, Zhu J, et al. Low health literacy predicts misperceptions of diabetes control in patients with persistently elevated A1C. Diabetes Educ. 2015; 41:309–319. PMID:
25699568.

137. McNaughton CD, Jacobson TA, Kripalani S. Low literacy is associated with uncontrolled blood pressure in primary care patients with hypertension and heart disease. Patient Educ Couns. 2014; 96:165–170. PMID:
24882088.

138. Stewart DW, Cano MA, Correa-Fernández V, et al. Lower health literacy predicts smoking relapse among racially/ethnically diverse smokers with low socioeconomic status. BMC Public Health. 2014; 14:716. PMID:
25018151.

139. James DC, Harville C, Efunbumi O, Martin MY. Health literacy issues surrounding weight management among African American women: a mixed methods study. J Hum Nutr Diet. 2015; 28(Suppl 2):41–49. PMID:
24890122.

140. Wolf MS, Davis TC, Curtis LM, et al. A patient-centered prescription drug label to promote appropriate medication use and adherence. J Gen Intern Med. 2016; 31:1482–1489. PMID:
27542666.

141. Yeung DL, Alvarez KS, Quinones ME, et al. Low-health literacy flashcards & mobile video reinforcement to improve medication adherence in patients on oral diabetes, heart failure, and hypertension medications. J Am Pharm Assoc (2003). 2017; 57:30–37. PMID:
27816544.
142. Rothman RL, DeWalt DA, Malone R, et al. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. JAMA. 2004; 292:1711–1716. PMID:
15479936.

143. Zoellner JM, Hedrick VE, You W, et al. Effects of a behavioral and health literacy intervention to reduce sugar-sweetened beverages: a randomized-controlled trial. Int J Behav Nutr Phys Act. 2016; 13:38. PMID:
27000402.

144. Burke LE, Ma J, Azar KM, et al. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2015; 132:1157–1213. PMID:
26271892.
145. Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017; 177:624–631. PMID:
28241271.
146. Christensen A, Christrup LL, Fabricius PE, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010; 28:194–200. PMID:
19770778.

147. Kooy MJ, van Wijk BL, Heerdink ER, de Boer A, Bouvy ML. Does the use of an electronic reminder device with or without counseling improve adherence to lipid-lowering treatment? The results of a randomized controlled trial. Front Pharmacol. 2013; 4:69. PMID:
23755014.

148. Varleta P, Acevedo M, Akel C, et al. Mobile phone text messaging improves antihypertensive drug adherence in the community. J Clin Hypertens (Greenwich). 2017; 19:1276–1284. PMID:
28941056.

149. Palmer MJ, Barnard S, Perel P, Free C. Mobile phone-based interventions for improving adherence to medication prescribed for the primary prevention of cardiovascular disease in adults. Cochrane Database Syst Rev. 2018; 6:CD012675. PMID:
29932455.

150. Cole-Lewis H, Kershaw T. Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010; 32:56–69. PMID:
20354039.

151. Free C, Knight R, Robertson S, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet. 2011; 378:49–55. PMID:
21722952.

152. Haapala I, Barengo NC, Biggs S, Surakka L, Manninen P. Weight loss by mobile phone: a 1-year effectiveness study. Public Health Nutr. 2009; 12:2382–2391. PMID:
19323865.

153. Whittaker R, Borland R, Bullen C, Lin RB, McRobbie H, Rodgers A. Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev. 2009; CD006611. PMID:
19821377.

154. Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med. 2016; 176:340–349. PMID:
26831740.
155. Buis L, Hirzel L, Dawood RM, et al. Text messaging to improve hypertension medication adherence in African Americans from primary care and emergency department settings: results from two randomized feasibility studies. JMIR Mhealth Uhealth. 2017; 5:e9. PMID:
28148474.

156. Bobrow K, Farmer AJ, Springer D, et al. Mobile phone text messages to support treatment adherence in adults with high blood pressure (SMS-Text Adherence Support [StAR]): a single-blind, randomized trial. Circulation. 2016; 133:592–600. PMID:
26769742.
157. Toma T, Athanasiou T, Harling L, Darzi A, Ashrafian H. Online social networking services in the management of patients with diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2014; 106:200–211. PMID:
25043399.

158. Gandapur Y, Kianoush S, Kelli HM, et al. The role of mHealth for improving medication adherence in patients with cardiovascular disease: a systematic review. Eur Heart J Qual Care Clin Outcomes. 2016; 2:237–244. PMID:
29474713.

159. Peiris D, Praveen D, Johnson C, Mogulluru K. Use of mHealth systems and tools for non-communicable diseases in low- and middle-income countries: a systematic review. J Cardiovasc Transl Res. 2014; 7:677–691. PMID:
25209729.

160. Davidson TM, McGillicuddy J, Mueller M, et al. Evaluation of an mHealth medication regimen self-management program for African American and Hispanic uncontrolled hypertensives. J Pers Med. 2015; 5:389–405. PMID:
26593951.
161. Free C, Phillips G, Galli L, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013; 10:e1001362. PMID:
23349621.

162. Tian M, Ajay VS, Dunzhu D, et al. A cluster-randomized, controlled trial of a simplified multifaceted management program for individuals at high cardiovascular risk (SimCard Trial) in Rural Tibet, China, and Haryana, India. Circulation. 2015; 132:815–824. PMID:
26187183.

163. Andre N, Wibawanti R, Siswanto BB. Mobile phone-based intervention in hypertension management. Int J Hypertens. 2019; 2019:9021017. PMID:
31080670.

164. Morawski K, Ghazinouri R, Krumme A, et al. Association of a smartphone application with medication adherence and blood pressure control: the MedISAFE-BP randomized clinical trial. JAMA Intern Med. 2018; 178:802–809. PMID:
29710289.
165. Ahnis A, Riedl A, Figura A, Steinhagen-Thiessen E, Liebl ME, Klapp BF. Psychological and sociodemographic predictors of premature discontinuation of a 1-year multimodal outpatient weight-reduction program: an attrition analysis. Patient Prefer Adherence. 2012; 6:165–177. PMID:
22442628.

166. Emery CF, Hauck ER, Blumenthal JA. Exercise adherence or maintenance among older adults: 1-year follow-up study. Psychol Aging. 1992; 7:466–470. PMID:
1388868.

167. Susin N, de Melo Boff R, Ludwig MW, et al. Predictors of adherence in a prevention program for patients with metabolic syndrome. J Health Psychol. 2016; 21:2156–2167. PMID:
25805660.

168. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000; 160:2101–2107. PMID:
10904452.
169. Eze-Nliam CM, Thombs BD, Lima BB, Smith CG, Ziegelstein RC. The association of depression with adherence to antihypertensive medications: a systematic review. J Hypertens. 2010; 28:1785–1795. PMID:
20531223.

170. Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011; 26:1175–1182. PMID:
21533823.

171. Pizzi C, Rutjes AW, Costa GM, Fontana F, Mezzetti A, Manzoli L. Meta-analysis of selective serotonin reuptake inhibitors in patients with depression and coronary heart disease. Am J Cardiol. 2011; 107:972–979. PMID:
21256471.

172. Morgan SG, Lee A. Cost-related non-adherence to prescribed medicines among older adults: a cross-sectional analysis of a survey in 11 developed countries. BMJ Open. 2017; 7:e014287.

173. Briesacher BA, Limcangco MR, Frech-Tamas F. New-user persistence with antihypertensives and prescription drug cost-sharing. J Clin Hypertens (Greenwich). 2007; 9:831–836. PMID:
17978589.

174. Heisler M, Langa KM, Eby EL, Fendrick AM, Kabeto MU, Piette JD. The health effects of restricting prescription medication use because of cost. Med Care. 2004; 42:626–634. PMID:
15213486.

175. Kamal-Bahl S, Briesacher B. How do incentive-based formularies influence drug selection and spending for hypertension? Health Aff (Millwood). 2004; 23:227–236. PMID:
15002647.

176. Karter AJ, Parker MM, Solomon MD, et al. Effect of out-of-pocket cost on medication initiation, adherence, and persistence among patients with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2018; 53:1227–1247. PMID:
28474736.

177. Maciejewski ML, Bryson CL, Perkins M, et al. Increasing copayments and adherence to diabetes, hypertension, and hyperlipidemic medications. Am J Manag Care. 2010; 16:e20–e34. PMID:
20059288.
178. Soumerai SB, Pierre-Jacques M, Zhang F, et al. Cost-related medication nonadherence among elderly and disabled Medicare beneficiaries: a national survey 1 year before the Medicare drug benefit. Arch Intern Med. 2006; 166:1829–1835. PMID:
17000938.
179. Taira DA, Wong KS, Frech-Tamas F, Chung RS. Copayment level and compliance with antihypertensive medication: analysis and policy implications for managed care. Am J Manag Care. 2006; 12:678–683. PMID:
17090224.
180. Després F, Forget A, Kettani FZ, Blais L. Impact of patient reimbursement timing and patient out-of-pocket expenses on medication adherence in patients covered by private drug insurance plans. J Manag Care Spec Pharm. 2016; 22:539–547. PMID:
27123915.

181. Yoon J, Ettner SL. Cost-sharing and adherence to antihypertensives for low and high adherers. Am J Manag Care. 2009; 15:833–840. PMID:
19895188.
182. Sedjo RL, Cox ER. Lowering copayments: impact of simvastatin patent expiration on patient adherence. Am J Manag Care. 2008; 14:813–818. PMID:
19067498.
183. Shrank WH, Hoang T, Ettner SL, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med. 2006; 166:332–337. PMID:
16476874.
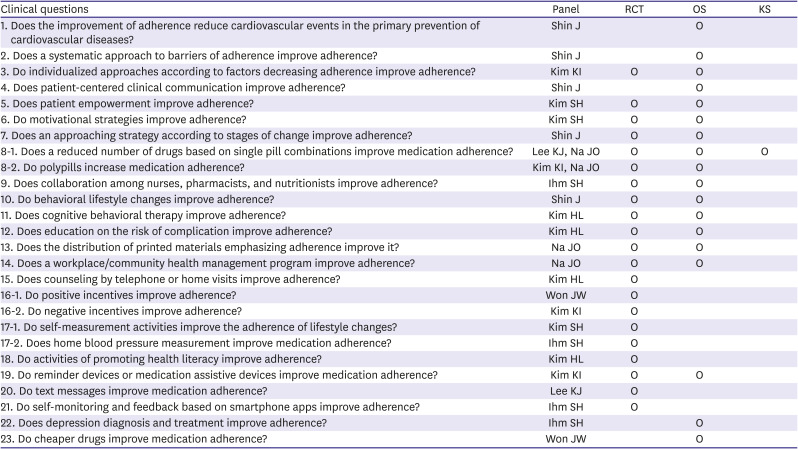

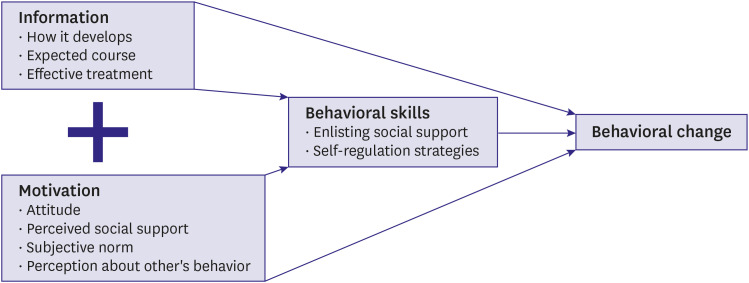
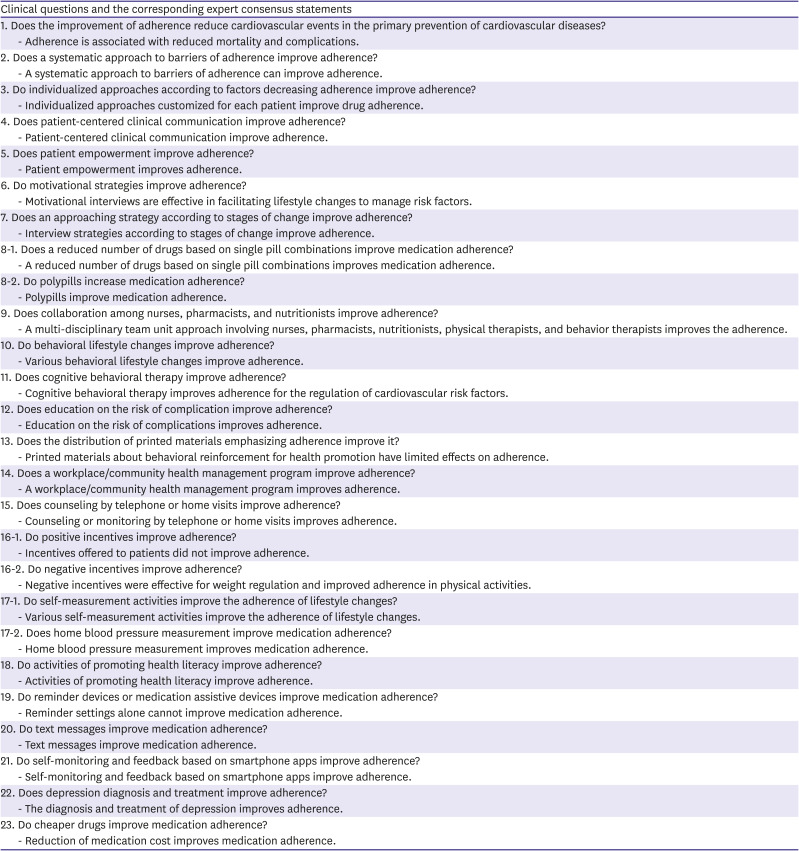




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download