INTRODUCTION
Macrosomia or high birth weight infant refers to a newborn with a birth weight ≥ 4 kg, regardless of gestational age. The incidence of macrosomia is approximately 3–7%, and about 1% of babies weigh ≥ 4.5 kg.
1 According to birth registration data from Statistics Korea, the incidence of macrosomia was 2.8% in 2019, which shows a decreasing trend compared to previous years, such as 3.6% in 2010.
23 Around the same time, the prevalence of macrosomia in developed countries was about 5–20%, which is increasing, and the situation in developing countries is also increasing, primarily due to the increasing obesity and diabetic disorders of reproductive-aged women.
4
The major risk factors for macrosomia are diabetic disease, maternal obesity, and family history of macrosomia. The prevalence of macrosomia is 13.3% among mothers with gestational diabetes mellitus (GDM) and 3.6% among non-GDM mothers. Around 6–10% of pregnant women are obese, and 19% of obese mothers are diabetic.
2 In addition, hypertension, multiparity, excessive maternal weight gain during pregnancy, advanced maternal age, and male fetus are often regarded as risk factors for macrosomia.
56
Macrosomia is associated with several maternal, perinatal, and neonatal complications, such as emergency cesarean section and injury to the birth canal and baby.
2456 Congenital anomalies are more common, and cardiac defects occur in the majority. Macrosomias are more prone to growth and developmental retardation, hypoglycemia, hypocalcemia, polycythemia, and hyperbilirubinemia. Research regarding the complications of low birth weight infants is varied, and great advancement in understanding and outcome has been achieved. However, there are fewer studies regarding macrosomia, especially on the national incidences and outcomes in Korea.
2 Only several single-center observational studies and one national epidemiologic analysis in 1996 are available.
The aim of this study was to estimate the incidence of macrosomia in Korea and to identify the growth and developmental outcomes and other neonatal complications that occur with macrosomia.
DISCUSSION
Macrosomia can be an important risk factor for maternal, perinatal, and neonatal complications. To the best of our knowledge, this is the most recent, nationwide epidemiologic study to consider the incidence and growth and developmental outcomes of macrosomia. This study showed macrosomia remains an issue due to persisting high BMI and poor developmental outcomes, which may lead to complications in adulthood. This study may be a great answer to the big, current health issue concerning childhood or adult metabolic syndrome, and may formulate treatment and prevention strategies. If we focus on this somewhat overlooked high-risk group, we may be able to improve their health and reduce enormous socioeconomic burden.
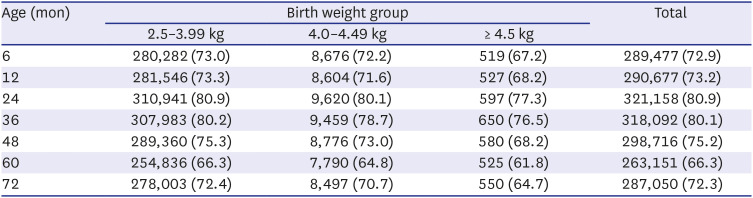
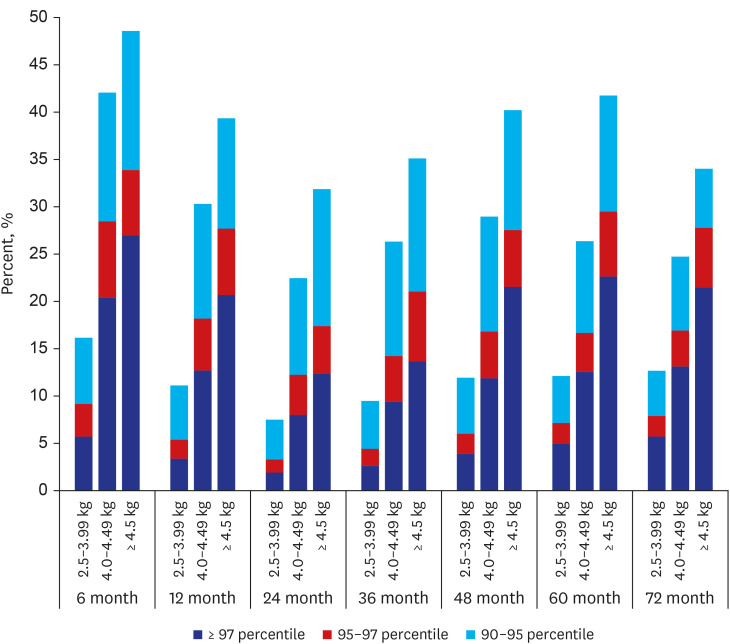
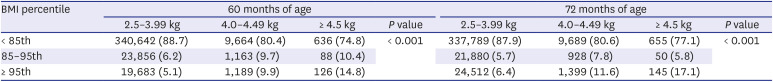
According to statistics from the Korean National Statistical Office, the frequency of macrosomia decreased annually from 6.7% to 2.8% between 1993 and 2019. This is thought to have been due to the improvement of prenatal maternal care, particularly the improvement of gestational diabetes treatment, which is the most important cause of macrosomia. In the past, about 60% of babies born to diabetic mothers were above the 95th percentile of the same age group in body weight, but this has recently decreased to 20–30%, due to improvements in gestational diabetes treatment.
10 Cho et al.
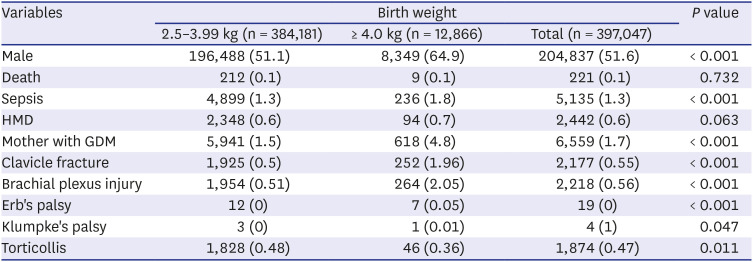
11 noted that GDM mothers are significantly more likely to deliver an infant with macrosomia. The frequency of macrosomia was 13.3% in the case of gestational diabetes but only 3.6% in mothers without gestational diabetes.
11 In this study, 4.8% of macrosomia had a mother with gestational diabetes, showing that GDM as a significant risk factor for macrosomia.
A study revealed that fetal macrosomia had an incidence of 9.1%.
12 A retrospective cohort study conducted at a large maternity unit in the UK between January 2009 and December 2016 showed the incidence of fetal macrosomia as 12.0%.
13 Maternal BMI and diabetes are strong risk factors for macrosomia, irrespective of region, in 23 developing countries in Africa, Asia, and Latin America.
8 Because infants with macrosomia show complications, a national study in the United Sates categorized macrosomia as > 4 kg, > 4.5 kg, and > 5 kg to better predict its risks.
14 For our study, we stratified macrosomia in the same manner.
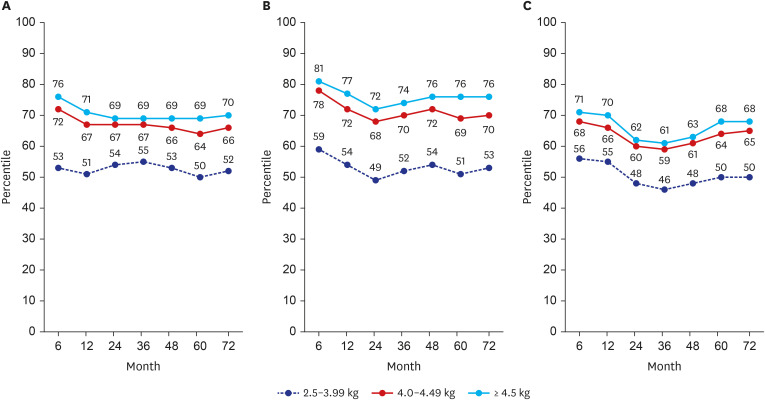
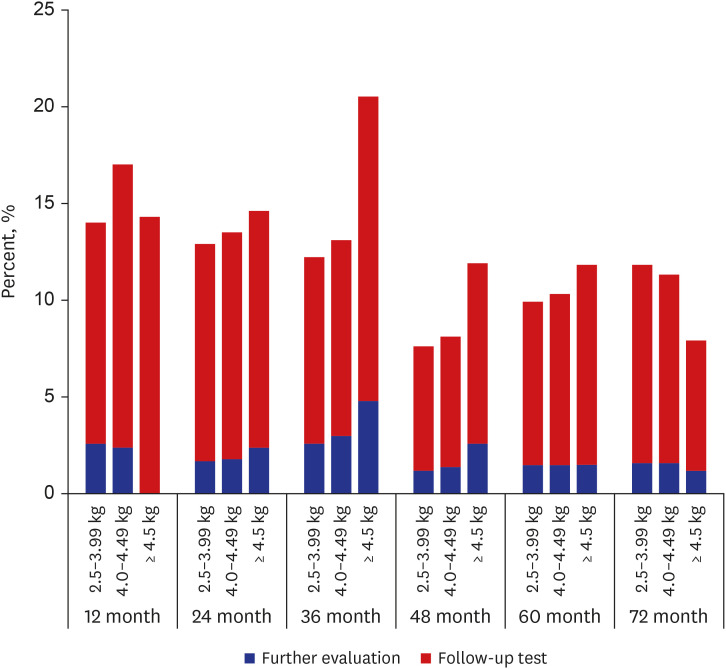
Postnatal catch-down growth in large for gestational age (LGA) or macrosomia may be protective against obesity and being overweight. LGA children without catch-down growth had an increased risk of overweight at 4 years of age.
15 In a longitudinal growth study of 2,465 Chinese children, catch-down growth in weight, length, and ponderal index of high birth weight term infants was not achieved within 12 months of age.
16 Peters et al.
17 showed in their study with LGA-children of mothers without GDM that 88% showed catch-down growth within 12 months of age. Despite the catch-down growth, the differences in the mean weight, length, and HC remained until 4 years of age, and those without catch-down growth had more subcutaneous fat. Irrespective of feeding pattern, the majority showed catch-down growth. However, the catch-down could not achieve complete normalization.
17 This is in line with our results, in which complete catch-down growth did not occur. Those with macrosomia remained within their growth percentile until 72 months of age. Considering these, prenatal treatment, such as GDM control and maternal weight control, is the most important factor in growth outcomes.
In meta-analysis, high birth weight or macrosomia is an independent risk factor for later overweight and obesity.
18 Obese children are more likely (up to 80%) to become obese adults.
19 This is consistent with our study results that BMI is consistently higher until 72 months of age in the macrosomia group. By considering that birth weight is essentially determined by in-utero condition, preventing in-utero over-nutrition by avoiding maternal overweight and/or controlling gestational diabetes will help to avoid overweight in the newborn and during childhood.
Several studies show that male gender is an independent risk factor for childhood overweight and obesity.
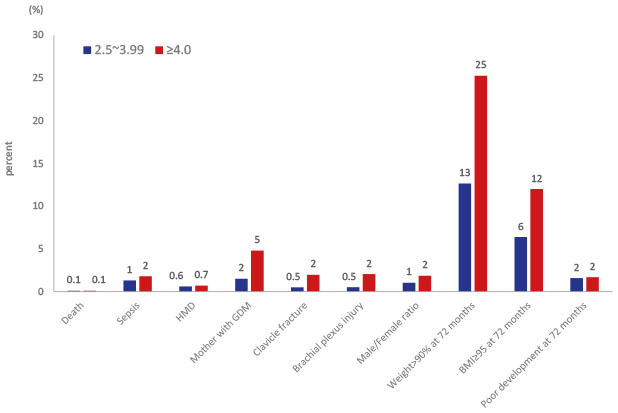
2021 In our study, male sex was an independent risk factor for macrosomia, and macrosomia was associated with an increased incidence of sepsis. Among infants with macrosomia, hyaline membrane disease is more common, possibly due to maternal diabetes.
22 Macrosomia was associated with increased mortality (OR, 2.2), and maternal diabetes and high birth weight showed synergy in mortality.
2324 In our study, there was no significant difference in HMD and mortality. In a comparative study between large infants of non-diabetic mothers and a control group of infants with 3–3.99 kg birth weight, clavicular fracture, Erb's palsy, and humerus fracture were significantly more frequent in the large infant group.
16 In our study, there were higher incidence of clavicular fracture, Erb's palsy, or brachial plexus palsy in the macrosomia group (2.1%) compared to in control group (0.5%).
There is a meta-analysis that statistically significant cognitive impairment in infants of diabetic mothers during the first year may result in a delay in mental performance afterwards.
25 In an experimental rat study, maternal obesity associated GDM resulted in neuroinflammation by microglial activation in the newborn offspring, and the influence sustained to young adulthood by astrogliosis and derangement of the hippocampal layer.
26 Generally, our study is consistent with previous studies showing the risks and poor outcomes of macrosomia. Infants with macrosomia are prone to neonatal disease, neurodevelopmental delay, and childhood overweight and obesity, particularly in boys.
Our study has several potential limitations. First, we could not be analyzed to determine a cause-effect relationship as an epidemiologic study. Second, a lack of familial background data, such as maternal and paternal height and BMI, and environmental factors like socioeconomic status and feeding pattern were not considered in this study due to the lack of data. Further studies need to incorporate these factors. Third, since the infants were included in only about 96.4% of the birth statistics, and the number of infants participated in the program was varied, and the weight was based on the questionnaire of the national health screening program for infants and children, the missing data or selection issue may potentially influence our findings. Last, diagnosis of several morbidities was identified according to ICD-10 codes of the NHIS claims database without reviewing the detailed clinical charts. The misclassification or omission have potentially affected our findings
In conclusion, macrosomia can be a risk factor for neonatal and childhood complications including obesity and developmental delay. Careful monitoring and proper strategies for prenatal care, and postnatal feeding and physical activity for catch-down growth may be helpful to prevent later adverse results.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download