Abstract
Public interventions have shown to optimize the use of antibiotics in children with acute otitis media (AOM). In this study, we describe the AOM-related antibiotic use among children in South Korea using national cohort data. We retrieved the Health Insurance Review & Assessment Service data to construct a national cohort of children aged 0–6 years who had been diagnosed with AOM between 2012 and 2018. Of 25,212,264 children included, the antibiotic prescription has increased for amoxicillin/amoxicillin-clavulanate from 56.1% in 2012 to 61.8% in 2018. Prescription has decreased for cephalosporin (35.1% in 2012 to 31.8% in 2018) and macrolide (8.7% in 2012 to 6.4% in 2018). National cohort data have shown an increased trend in AOM-related aminopenicillin prescription and downward trend cephalosporin and macrolide use in South Korea. A multi-faceted approach is required to control the antimicrobial resistance at a population level.
Graphical Abstract
Acute otitis media (AOM) is the leading diagnosis for antibiotic prescribing in children.1 Nonetheless, misuse of antibiotics promotes antimicrobial resistance and adverse effects in patients. Aligning with the Global Action Plan on Antimicrobial Resistance endorsed by the World Health Assembly in 2015, many countries have adopted national action plans on antimicrobial resistance (NAP-AMR) that include quality evaluation programs of antibiotic use in outpatient setting.2 The goal of Korean NAP-AMR include 1) promoting prudent use of antimicrobial medicines; 2) prevent the spread of AMR; 3) strengthen the surveillance system; 4) improve awareness; 5) strengthen research and development; and 6) enhance international collaboration.2 In such context, the governmental and financial support on research has increased significantly.3 To date, public interventions have shown to optimize the use of antibiotics in children with AOM, however, it has not been clearly demonstrated in countries with high level of antibiotic consumption.4
Characterized by the universal health coverage, South Korea has an equitable access to healthcare safety net while the level of antibiotic consumption is among the highest in the high-income countries.5 The outline of South Korean NAP-AMR has been developed in between 2012–2015 which includes monitoring and surveillance, guideline development, reimbursement policy, and support of antimicrobial stewardship in different clinical settings. The universal health coverage in Korea provides a unique opportunity to observe the trend of antibiotic prescription pattern in children with AOM at a national level. In this study, we describe the AOM-related antibiotic use among children in South Korea using national cohort data.
The National health insurance system of South Korea is a public, single-payer system covering > 99% of all citizens and residents. Following the medical services, the provider send reimbursement claims for medical expenses incurred to the Health Insurance Review & Assessment Service (HIRA), which then reviews claims, assesses the quality of care provided, and evaluates healthcare services' adequacy. As a part of a Joint Project on Quality Assessment Research (No. M20200211292), we retrieved the HIRA data to construct a national cohort of children aged 0–6 years who had been diagnosed with AOM between January 2012 and December 2018.
A case with AOM was identified based on the presence of International Classification of Diseases, 10th revision, Clinical Modification (ICD-10-CM) diagnosis code indicating AOM and their subcodes for reimbursement purpose (Supplementary Table 1). We defined AOM cases which needs antibiotic treatment when the case is with both of 1) AOM diagnostic code and 2) any type of antibiotics prescription. All visits with any of the diagnostic codes of AOM in children under 6 years of age was counted as a single episode. AOM visits occurring within the same month were considered as follow-up visits and therefore not counted as another episode. AOM visits occurring after the calendar month were considered as separate episode. Information on antibiotics prescription was retrieved from treatment codes which include generic name of antibiotics. We coded the antibiotics as one of the 3 classes: 1) amoxicillin/amoxicillin-clavulanate, 2) cephalosporin, and 3) macrolide. We excluded other antibiotics from this study.
To estimate the trend difference over time after controlling for potential confounding effects, we included child's sex, age groups (0–23 months vs. 2–6 years), types of AOM (suppurative AOM vs. otitis media with effusion), level of hospital (primary, secondary, tertiary), and regions (capital area vs. non-capital area) into the model.
This study consists of four analyses: First, we calculated descriptive statistics for changing proportion of antibiotics class used and difference in proportions of each antibiotic groups between 2012–2015 and 2016–2018. Change in annual proportion of three antibiotics was tested for linear trend using Kendall tau test. Second, we examined the pattern of antibiotic prescription stratified by age groups, types of AOM, hospital and regions (capital area vs. non-capital area). Third, we conducted a log-binomial logistic regression model to calculate adjusted risk ratios (aRR) of antibiotic use considering all covariates. We used SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
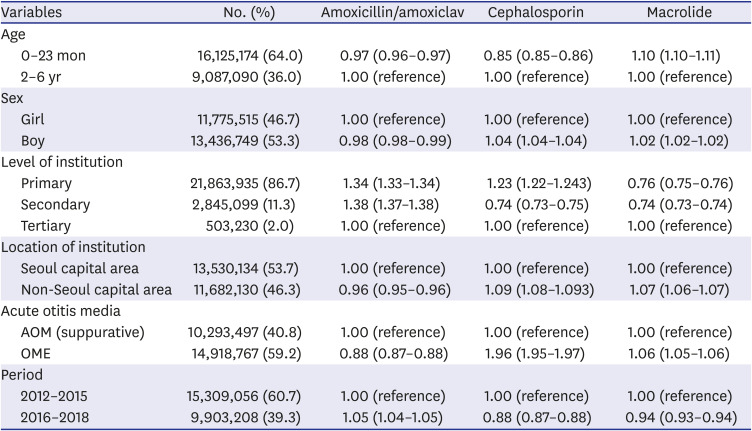
Table 1 shows the baseline characteristic of children prescribed with antibiotics for AOM between 2012 and 2018 in South Korea. Of 25,212,264 children included, 64.0% were aged 0–23 months, 53.3% were boys, 53.7% were prescribed from Seoul capital area, 86.7% were prescribed from primary care clinics, 40.8% were diagnosed with suppurative AOM, and 59.9% were prescribed with amoxicillin/amoxicillin-clavulanate. Younger age (0–23 months) was associated with lower probability of prescription with amoxicillin/amoxicillin-clavulanate (aRR, 0.97; 95% confidence interval [CI], 0.96–0.97) and cephalosporin (aRR, 0.85; 95% CI, 0.85–0.86), while had higher probability of have prescribed with macrolide (aRR, 1.10; 95% CI, 1.10–1.11). Primary (aRR, 1.34; 95% CI, 1.33–1.34) and secondary (aRR, 1.38; 95% CI, 1.37–1.38) healthcare services had higher probability of prescribing amoxicillin/amoxicillin-clavulanate than tertiary centers, while had lower probability for prescribing macrolides (aRR, 0.76 for primary care; aRR, 0.74 for secondary care services). Amoxicillin/amoxicillin-clavulanate was more likely to be prescribed for suppurative AOM, while cephalosporin and macrolide were more likely to be prescribed for nonsuppurative AOM. Between 2012–2015 vs. 2016–2018, amoxicillin/amoxicillin-clavulanate had higher probability of prescription (aRR, 1.05; 95% CI, 1.04–1.05), while cephalosporin (aRR, 0.88; 95% CI, 0.87–0.88) and macrolide (aRR, 0.94; 95% CI, 0.93–0.94) had lower probability of prescription.
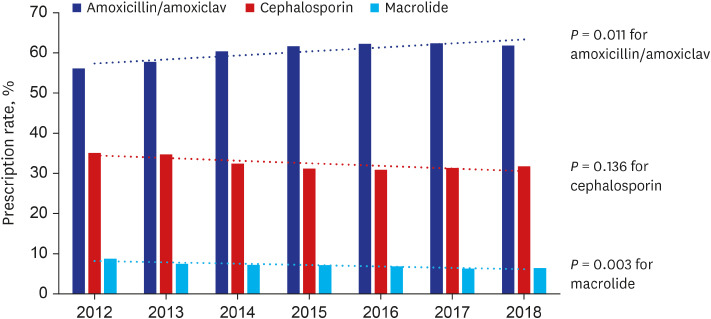
Fig. 1 depicts trend in antibiotic prescription pattern between 2012 and 2018. The proportion of antibiotic prescription has increased for amoxicillin/amoxicillin-clavulanate from 56.1% in 2012 to 61.8% in 2018 (P = 0.011). On another hand, the prescription proportion has decreased for cephalosporin (35.1% in 2012 to 31.8% in 2018, P = 0.136) and macrolide (8.7% in 2012 to 6.4% in 2018, P = 0.003).
Our analysis of national cohort data for 7-year period found a significant increase in AOM-related prescription of aminopenicillins (from 56.1% to 61.8%, P = 0.011), while a substantial decrease in macrolide use in children was also noted (from 8.7% to 6.4%, P = 0.003). To our knowledge, this is the first study to determine AOM-related antibiotic use trends in the setting of NAP-AMR at national level. The national trend was assessed in different levels in other countries. In Japan, following the introduction of NAP-AMR in 2016, the total use of antimicrobials and broad-spectrum antibiotics such as cephalosporins, fluoroquinolones, and macrolides have declined.6 In Italy, the consumption of antibiotics has decreased from 18.4 daily defined dose (DDD) in 2013 to 16.1 DDD in 2018 following adopting NAP-AMR.7 In line with South Korean NAP-AMR, the HIRA has been conducting annual review on the antibiotic prescription data of all insurers.8 Assessment on the appropriateness of antibiotics use is made based on pediatric AOM guideline, which was developed in 2010.9 The guideline addresses the diagnosis and management of AOM including choice of antibiotics, with having amoxicillin or amoxicillin-clavulanate as a first-line drug of choice in most case scenarios.
It is surprising to find that the children aged 2–6 years old, who are recommended to withhold antibiotics according to the current Korean guideline, comprised 36.0% of the studied population.9 This is in line with finding from elsewhere, such as in the U.S., where 98.0% of children ≥ 2 years of age with AOM were prescribed an antibiotic in 2018.10 Although the trend by age group was not assessed in this study, the finding suggests a more judicious use of antibiotics should be directed especially for the preschool aged children above 2 years of age.
We also found the differential prescription pattern depending on the institutional factors (location, level). The regional difference in antibiotic use pattern for AOM may be attributable to the region-specific disease burden, which we were not able to analyze in the present study.11 As in previous study, healthcare claims can be useful to identify populations and providers with high antibiotic use and could be used to track and report antibiotic prescribing frequency.12 Studies to explore the barriers to appropriate prescribing of antibiotics should be in place in near future.
There are several limitations of this study. First, completeness and accuracy of case ascertainment is the intrinsic limitation of claims data. We classified suppurative vs. non-suppurative AOM according to the ICD-10 codes, however, there are limitations in case ascertainment. Second, individual data on other recognized risk (i.e., co-infections) or protective factors were not available. Lastly, we were unable to determine the regimen and within-class antibiotics (i.e., distinguishing between amoxicillin vs. amoxicillin-clavulanate, duration of therapy, dosage) prescribed to children who received prescriptions.
Despite these limitations, this is the first study to evaluate the policy for an entire defined population at a national level on antibiotic use in childhood AOM. HIRA has championed national efforts to address antibiotic overuse and misuse through quality evaluation and monitoring. This study did not address the direct effect of NAP-AMR on antibiotic resistance rate, however the decline in usage of cephalosporin and macrolide likely will reduce the antibiotic pressure at a population level thus to contribute to AMR control.
In conclusion, national cohort data have shown a continuous downward trend in AOM-related broad-spectrum antibiotic use from 2012 to 2018 amid introduction of NAP-AMR in South Korea. Given the magnitude of antibiotics prescribed for AOM in children, a multi-faceted approach will be needed to control the antimicrobial resistance at a population level.
Notes
Funding: This study was supported by the Joint Project on Quality Assessment Research using Health Insurance Review & Assessment Service data, and the Korea University Research Fund (K2025301, K2022961).
Author Contributions:
Conceptualization: Choe YJ.
Data curation: Park KH, Choe SA, Choe YJ.
Formal analysis: Choe SA, Choe YJ.
Investigation: Park KH, Shin JY.
Methodology: Park KH, Shin J, Choe YJ.
Supervision: Choe YJ.
Validation: Shin J.
Writing - original draft: Park KH, Choe SA, Choe YJ.
Writing - review & editing: Park KH, Choe SA, Shin J, Choe YJ.
References
1. Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev. 2015; (6):CD000219. PMID: 26099233.

2. Ryu S. The new Korean action plan for containment of antimicrobial resistance. J Glob Antimicrob Resist. 2017; 8:70–73. PMID: 28024981.

3. Ryu S, Head MG, Kim BI, Hwang J, Cho EH. Are we investing wisely? A systematic analysis of nationally funded antimicrobial resistance projects in Republic of Korea, 2003–2013. J Glob Antimicrob Resist. 2016; 6:90–94. PMID: 27530848.

4. Marom T, Tan A, Wilkinson GS, Pierson KS, Freeman JL, Chonmaitree T. Trends in otitis media-related health care use in the United States, 2001–2011. JAMA Pediatr. 2014; 168(1):68–75. PMID: 24276262.

5. Choe YJ, Shin JY. Trends in the use of antibiotics among Korean children. Korean J Pediatr. 2019; 62(4):113–118. PMID: 30852884.

6. Kusama Y, Tsuzuki S, Muraki Y, Koizumi R, Ishikane M, Ohmagari N. The effects of Japan's National Action Plan on antimicrobial resistance on antimicrobial use. Int J Infect Dis. 2021; 103:154–156. PMID: 33227519.

7. Cangini A, Fortinguerra F, Di Filippo A, Pierantozzi A, Da Cas R, Villa F, et al. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br J Clin Pharmacol. 2021; 87(3):1033–1042. PMID: 32643167.

8. Yun JM, Shin DW, Hwang SS, Cho J, Nam YS, Kim JH, et al. Effect of public disclosure on antibiotic prescription rate for upper respiratory tract infections. JAMA Intern Med. 2015; 175(3):445–447. PMID: 25506784.

9. Lee HJ, Park SK, Choi KY, Park SE, Chun YM, Kim KS, et al. Korean clinical practice guidelines: otitis media in children. J Korean Med Sci. 2012; 27(8):835–848. PMID: 22876048.

10. Frost HM, Becker LF, Knepper BC, Shihadeh KC, Jenkins TC. Antibiotic prescribing patterns for acute otitis media for children 2 years and older. J Pediatr. 2020; 220:109–115.e1. PMID: 32111379.
11. Howarth T, Brunette R, Davies T, Andrews RM, Patel BK, Tong S, et al. Antibiotic use for Australian Aboriginal children in three remote Northern Territory communities. PLoS One. 2020; 15(4):e0231798. PMID: 32302359.

12. Watson JR, Wang L, Klima J, Moore-Clingenpeel M, Gleeson S, Kelleher K, et al. Healthcare claims data: an underutilized tool for pediatric outpatient antimicrobial stewardship. Clin Infect Dis. 2017; 64(11):1479–1485. PMID: 28329388.

Fig. 1
Trend of antibiotic use for acute otitis media in children, Korean national cohort, 2012–2018.
amoxiclav = amoxicillin-clavulanate.
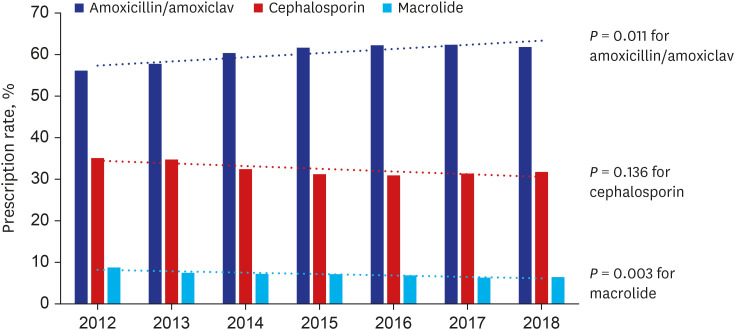
Table 1
Association between individual and institutional factors for probability of antibiotic prescription by classes among children with otitis media, national cohort, South Korea, 2012–2018




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download