The ongoing coronavirus disease 2019 (COVID-19) pandemic is one of the most significant public health threats in modern history and has changed many aspects of medical care and the daily lives of various people. Until recently, many of the response strategies against this pandemic have been based on previous experience with other highly pathogenic coronaviruses such as severe acute respiratory syndrome and Middle East respiratory syndrome.
12 As the virologic, epidemiologic, and clinical aspects of this emerging infectious disease are gradually revealed, the effects of severe acute respiratory syndrome coronavirus-2 on a possibly vulnerable subpopulation are currently investigated. The anatomic, physiologic, and immunologic changes that occur as a normal part of pregnancy may cause pregnant women to be more susceptible and vulnerable to viral infections.
3
However, the susceptibility of pregnant women to COVID-19 and related complications remains controversial and unresolved. Data on the clinical impact of COVID-19 on pregnant women in the Republic of Korea (ROK) are limited.
45678 This information is critical to assessing the risk-benefit of a COVID-19 vaccine, whose Food and Drug Administration approval for use among pregnant women is still pending. Therefore, this study aimed to investigate the clinical characteristics and outcomes in pregnant women diagnosed with COVID-19 during the early stage of the COVID-19 pandemic.
This observational multicenter cohort study was performed using a nationwide database obtained from the medical records from multiple Korean hospitals. In collaboration with Korea Disease Control and Prevention Agency (KDCA) and the National Medical Center, the demographic, epidemiological, and early clinical information of confirmed COVID-19 patients were collected from the government registry from January 2020 to April 2021. After extracting the relevant information from the KDCA database, we compared the maternal outcomes and clinical characteristics of pregnant women diagnosed with COVID-19 with those of non-pregnant women of childbearing age at the onset of the pandemic in Korea. All patients underwent reverse-transcription polymerase chain reaction (RT-PCR) to confirm the diagnosis of COVID-19.
Data on age, sex, body mass index (BMI), clinical symptoms at the time of diagnosis (pharyngitis, runny nose, myalgia, fatigue, dyspnea, headache, change of consciousness, nausea, and diarrhea), intensive care unit (ICU) admission, supplemental oxygen requirement, mechanical ventilation, multiple organ failure, death, comorbidities (diabetes, hypertension, chronic heart disease, asthma, chronic obstructive pulmonary disease, chronic kidney disease, cancer, chronic liver disease, autoimmune disease, and dementia), and complete blood count (CBC) were obtained. Statistical tests including χ2 test, Fisher's exact test, Mantel-Haenszel test, and Mann-Whitney U test were used according to the characteristics of the data.
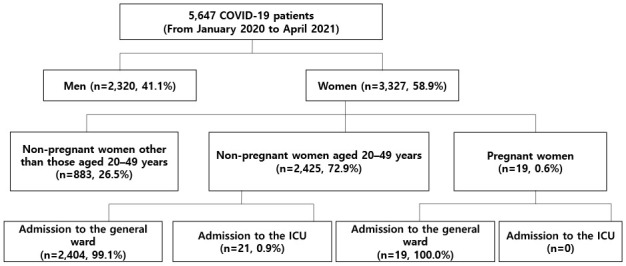
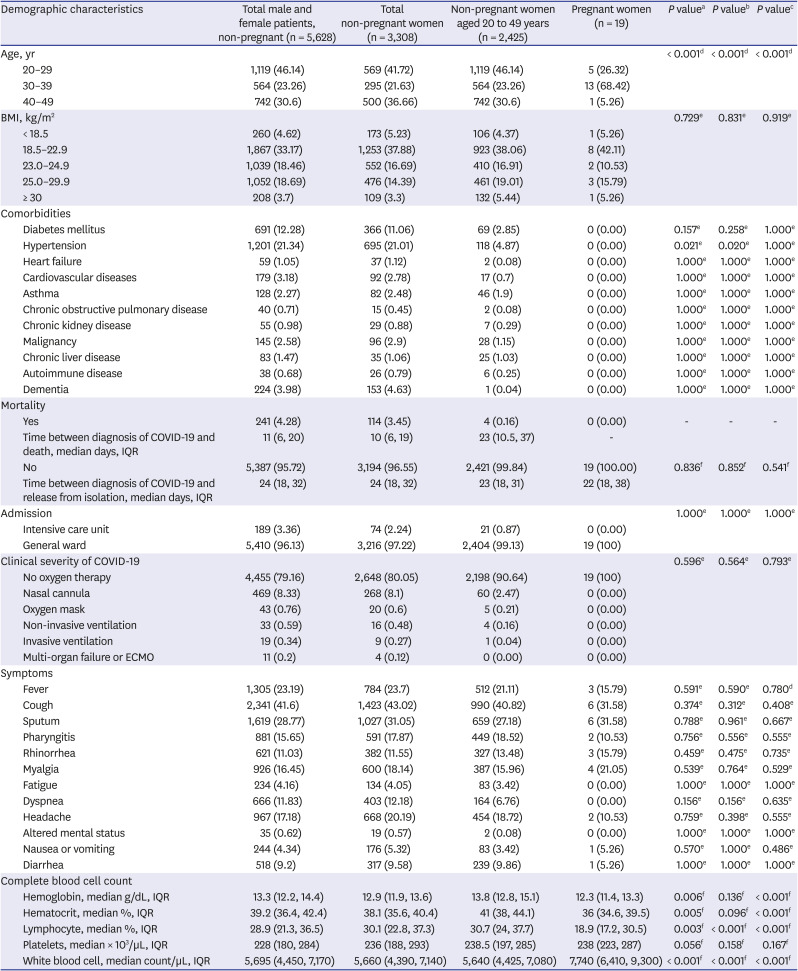
From January 2020 to April 2021, 5,647 COVID-19 patients were included in the registry. Among these patients, 3,327 (58.9%) were women, and 2,444 were women of childbearing age. A total of 19 patients were pregnant women: 5 (26.3%) were aged 20–29 years; 13 (68.4%) were aged 30–39 years, and 1 (5.3%) was aged 40–49 years.
The mortality rates among the total study patients and female patients were 4.3% and 3.5%, respectively. No deaths occurred among pregnant women, while four (0.16%) deaths occurred among women of childbearing age. The median period (interquartile range, IQR) from COVID-19 diagnosis to death in women of childbearing age was 23 (11–37) days. In all patients, the duration between diagnosis of COVID-19 and release from isolation was 23 (18–31) days or 24 (18–32) days for women of childbearing age, and 22 (18–38) days for pregnant women.
All pregnant women in the case group were admitted to the general ward, whereas 21 patients in the control group (0.87%) were admitted to the ICU (P = 1.000). None of the pregnant women required supplemental oxygen, while 70 non-pregnant women of childbearing age required supplemental oxygen with or without mechanical ventilation.
None of the pregnant women had comorbidities, while the control group had various pre-existing conditions (
Table 1). No significant difference was found in the clinical symptoms between the two groups (
Table 1). Six of the 19 pregnant women were asymptomatic.
Meanwhile, significant differences were observed in the CBC results between pregnant women and non-pregnant women: hemoglobin (g/dL) [median (IQR); 12.3 (11.4–13.3) vs. 13.8 (12.8–15.1), P < 0.001], white blood cell (WBC) count (cells/µL) [7,740 (6,410–9,300) vs. 5,640 (4,425–7,080), P < 0.001], and lymphocyte (%) [18.9 (17.2–30.5) vs. 30.7 (24.0–37.7), P < 0.001].
This observational multicenter study demonstrated that all 19 pregnant women with confirmed COVID-19 did not exhibit worsening of disease compared with that in non-pregnant women. To our knowledge, this is the first study to compare the morbidity and mortality rates of COVID-19 pregnant women with those of non-pregnant women of childbearing age in the ROK. Focusing on the clinical impact of COVID-19 during pregnancy can aid physicians and policy-makers in the rational prioritization of COVID-19 vaccination strategies and improving the management of COVID-19 cases. Specifically, the clinical data of domestic groups at higher risk for COVID-19 are critical for improving national preparedness and response.
Previous research provided contradictory findings regarding the increased risk for critical illness and mortality resulting from COVID-19 during pregnancy. The physiologic and immunologic changes that occur during pregnancy may increase pregnant women's vulnerability to severe infection. Previous studies suggested that pregnancy is associated with severe or critical COVID-19.
9101112 Considering the mortality rate in pregnant women with COVID-19 described in a Brazilian study, the effect of comorbid conditions and the specific viral strain may be more significant than the pregnancy status in determining clinical severity.
13 Previous observational studies noted that majority of pregnant patients do not experience severe or critical COVID-19.
614151617181920212223 Sentilhes et al.
24 reported that maternal age, obesity, hypertension, or diabetes may increase the risk in pregnant women with COVID-19. Because none of the pregnant women included in our study had comorbidities, further analysis in this regard was not possible.
In previous studies, the clinical outcomes associated with COVID-19 did not appear to be worse in pregnant women compared with those in the general population.
614151617181920212223 However, data are still limited and the sex disparities in clinical severity and mortality of COVID-19 may be a result of the sex differences in comorbidities and behaviors. Men with COVID-19 appear to have worse clinical outcomes than those in women with COVID-19.
25262728 Furthermore, older age is strongly associated with increased mortality in COVID-19 patients.
282930 Considering that women have better outcomes than those in men,
2728 the hormonal profile and associated physiologic changes in young pregnant women may prevent worsening of the disease. However, the physiologic reserves and immunity are reduced in this patient group.
Conflicting studies suggest that COVID-19 during pregnancy may or may not lead to an increased risk of pregnancy complications such as preeclampsia, preterm birth, miscarriage, and perinatal death.
17192131323334 Numerous factors including medical comorbidities, physiologic changes in pregnancy, and baseline susceptibility to infection in addition to social factors such as limited access to care and difficulties with social isolation may negatively impact both maternal and fetal outcomes. Further studies among Korean patients are required to explain the outcomes observed in our population.
In women with normal pregnancy, the leukocyte count was higher and the lymphocyte percentage was lower among pregnant women with COVID-19 than that among non-pregnant controls. A previous meta-analysis demonstrated a significant correlation between increased leukocyte count and decreased lymphocyte count among patients with severe cases of COVID-19 compared with those with mild cases.
35 However, these laboratory findings may not be associated with worsening disease, but may result from other benign processes. In severe cases of COVID-19, intensive inflammation may lead to destruction of lymphatic tissues, lymphocyte apoptosis, and direct lymphocyte infection, in addition to lymphocyte inhibition due to metabolic disorders, including lactic acidosis.
36 On the contrary, pregnancy may cause immunomodulation resulting in the recognition and destruction of similar self-antigens.
During the initial stage of the pandemic, pregnant women in the ROK were hospitalized and managed according to the national policy requiring inpatient isolation, unlike other countries. These measures were implemented to prevent the lack of prenatal care, surveillance, and adequate treatment for those with COVID-19. This policy may explain why the severity of infection was similar in pregnant and non-pregnant patients.
Our study has several limitations. This analysis only included a very small number of pregnant women who did not have underlying diseases or complications associated with pregnancy. Furthermore, our study did not investigate the delivery mode, gestational age at the time of delivery, and frequency of vertical transmission or neonatal outcomes due to the limited data extracted from the government-operated registry.
In conclusion, pregnant women with COVID-19 infection were not at higher risk of severe or critical disease that would require supplemental oxygen, ICU admission, or mechanical ventilation. None of the pregnant women in our cohort experienced severe infection sequelae including sepsis, multiple organ failure, or maternal mortality. However, further analysis of multinational pregnancy cohorts is warranted to improve our understanding and strategies of managing COVID-19 in pregnant women.
Ethics statement
The study protocol was approved by the Institutional Review Board (IRB) of Korea University Anam Hospital (IRB no. 2020AN0408). The need for obtaining informed consent was waived due to the retrospective nature of the study.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download