1. Ley B, Collard HR, King TE Jr, King J. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011; 183(4):431–440. PMID:
20935110.

2. Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015; 192(2):e3–19. PMID:
26177183.

3. Shah NR, Noble P, Jackson RM, King TE Jr, Nathan SD, Padilla M, et al. A critical assessment of treatment options for idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005; 22(3):167–174. PMID:
16315778.

4. Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003; 168(5):538–542. PMID:
12773325.

5. Flaherty KR, Mumford JA, Murray S, Kazerooni EA, Gross BH, Colby TV, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003; 168(5):543–548. PMID:
12773329.

6. Caminati A, Bianchi A, Cassandro R, Mirenda MR, Harari S. Walking distance on 6-MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med. 2009; 103(1):117–123. PMID:
18786822.

7. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26(2):319–338. PMID:
16055882.
8. McLean A, Warren PM, Gillooly M, MacNee W, Lamb D. Microscopic and macroscopic measurements of emphysema: relation to carbon monoxide gas transfer. Thorax. 1992; 47(3):144–149. PMID:
1519189.

9. Trip P, Nossent EJ, de Man FS, van den Berk IA, Boonstra A, Groepenhoff H, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013; 42(6):1575–1585. PMID:
23949959.

10. Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987; 22(4):487–497. PMID:
3501693.

11. Erasmus JJ, Patz EF Jr. Positron emission tomography imaging in the thorax. Clin Chest Med. 1999; 20(4):715–724. PMID:
10587793.

12. Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011; 31(1):3–13. PMID:
21245592.

13. Griffeth LK. Use of PET/CT scanning in cancer patients: technical and practical considerations. Proc (Bayl Univ Med Cent). 2005; 18(4):321–330. PMID:
16252023.

14. Groves AM, Win T, Screaton NJ, Berovic M, Endozo R, Booth H, et al. Idiopathic pulmonary fibrosis and diffuse parenchymal lung disease: implications from initial experience with 18F-FDG PET/CT. J Nucl Med. 2009; 50(4):538–545. PMID:
19289428.

15. El-Chemaly S, Malide D, Yao J, Nathan SD, Rosas IO, Gahl WA, et al. Glucose transporter-1 distribution in fibrotic lung disease: association with [
18F]-2-fluoro-2-deoxyglucose-PET scan uptake, inflammation, and neovascularization. Chest. 2013; 143(6):1685–1691. PMID:
23699745.
16. Lee EY, Wong CS, Fung SL, Yan PK, Ho JC. SUV as an adjunct in evaluating disease activity in idiopathic pulmonary fibrosis - a pilot study. Nucl Med Commun. 2014; 35(6):631–637. PMID:
24472818.

17. Nobashi T, Kubo T, Nakamoto Y, Handa T, Koyasu S, Ishimori T, et al. 18F-FDG uptake in less affected lung field provides prognostic stratification in patients with interstitial lung disease. J Nucl Med. 2016; 57(12):1899–1904. PMID:
27339874.

18. Justet A, Laurent-Bellue A, Thabut G, Dieudonné A, Debray MP, Borie R, et al. [
18F]FDG PET/CT predicts progression-free survival in patients with idiopathic pulmonary fibrosis. Respir Res. 2017; 18(1):74. PMID:
28449678.

19. Castiaux A, Van Simaeys G, Goldman S, Bondue B. Assessment of 18F-FDG uptake in idiopathic pulmonary fibrosis: influence of lung density changes. Eur J Hybrid Imaging. 2018; 2(1):27.

20. Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011; 365(12):1079–1087. PMID:
21992121.

21. Lee SH, Yeo Y, Kim TH, Lee HL, Lee JH, Park YB, et al. Korean guidelines for diagnosis and management of interstitial lung diseases: part 2. idiopathic pulmonary fibrosis. Tuberc Respir Dis (Seoul). 2019; 82(2):102–117. PMID:
30841014.

22. Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005; 26(3):511–522. PMID:
16135736.

23. Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005; 26(4):720–735. PMID:
16204605.

24. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166(1):111–117. PMID:
12091180.
25. Ley B, Ryerson CJ, Vittinghoff E, Ryu JH, Tomassetti S, Lee JS, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med. 2012; 156(10):684–691. PMID:
22586007.

26. Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016; 194(3):265–275. PMID:
27299520.

27. Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004; 45(9):1431–1434. PMID:
15347707.
28. Watanabe H, Kanematsu M, Goshima S, Kondo H, Kawada H, Noda Y, et al. Adrenal-to-liver SUV ratio is the best parameter for differentiation of adrenal metastases from adenomas using 18F-FDG PET/CT. Ann Nucl Med. 2013; 27(7):648–653. PMID:
23625579.

29. Lambrou T, Groves AM, Erlandsson K, Screaton N, Endozo R, Win T, et al. The importance of correction for tissue fraction effects in lung PET: preliminary findings. Eur J Nucl Med Mol Imaging. 2011; 38(12):2238–2246. PMID:
21874321.

30. Win T, Screaton NJ, Porter JC, Ganeshan B, Maher TM, Fraioli F, et al. Pulmonary
18F-FDG uptake helps refine current risk stratification in idiopathic pulmonary fibrosis (IPF). Eur J Nucl Med Mol Imaging. 2018; 45(5):806–815. PMID:
29335764.
31. Nathan SD, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, et al. Effect of continued treatment with pirfenidone following clinically meaningful declines in forced vital capacity: analysis of data from three phase 3 trials in patients with idiopathic pulmonary fibrosis. Thorax. 2016; 71(5):429–435. PMID:
26968970.

32. Nakazawa S, Shimizu K, Mogi A, Kuwano H. Low diffusing capacity, emphysema, or pulmonary fibrosis: who is truly pulling the lung cancer strings? J Thorac Dis. 2018; 10(2):600–602. PMID:
29607119.

33. Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med. 2004; 45(9):1519–1527. PMID:
15347719.
34. Bural GG, Torigian DA, Chen W, Houseni M, Basu S, Alavi A. Increased 18F-FDG uptake within the reticuloendothelial system in patients with active lung cancer on PET imaging may indicate activation of the systemic immune response. Hell J Nucl Med. 2010; 13(1):23–25. PMID:
20411166.
35. Liu G, Hu Y, Zhao Y, Yu H, Hu P, Shi H.Variations of the liver standardized uptake value in relation to background blood metabolism: an 2-[18F]Fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography study in a large population from China. Medicine. 2018; 97(19):e0699. PMID:
29742723.
36. Tomassetti S, Gurioli C, Ryu JH, Decker PA, Ravaglia C, Tantalocco P, et al. The impact of lung cancer on survival of idiopathic pulmonary fibrosis. Chest. 2015; 147(1):157–164. PMID:
25166895.

37. Lee HY, Cho J, Kwak N, Lee J, Park YS, Lee CH, et al. Prognostic impact of malignant diseases in idiopathic pulmonary fibrosis. Sci Rep. 2020; 10(1):18260. PMID:
33106517.

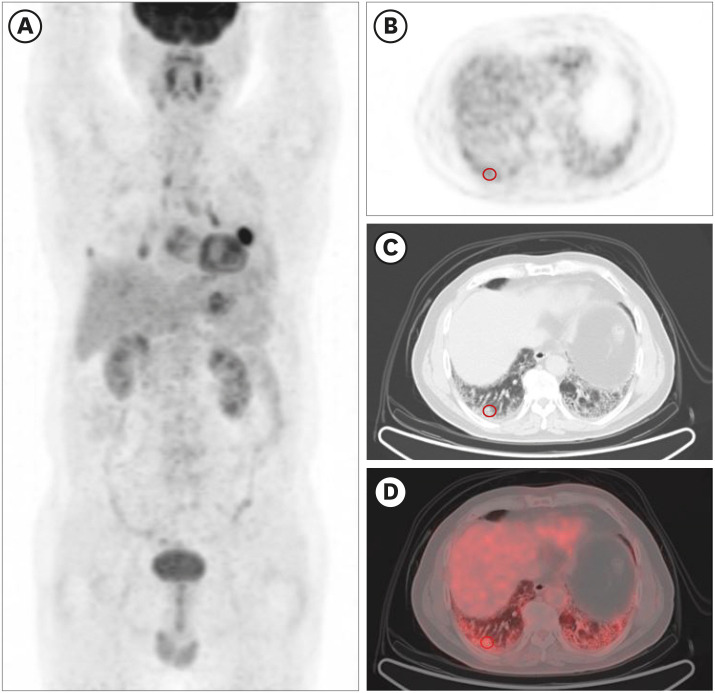
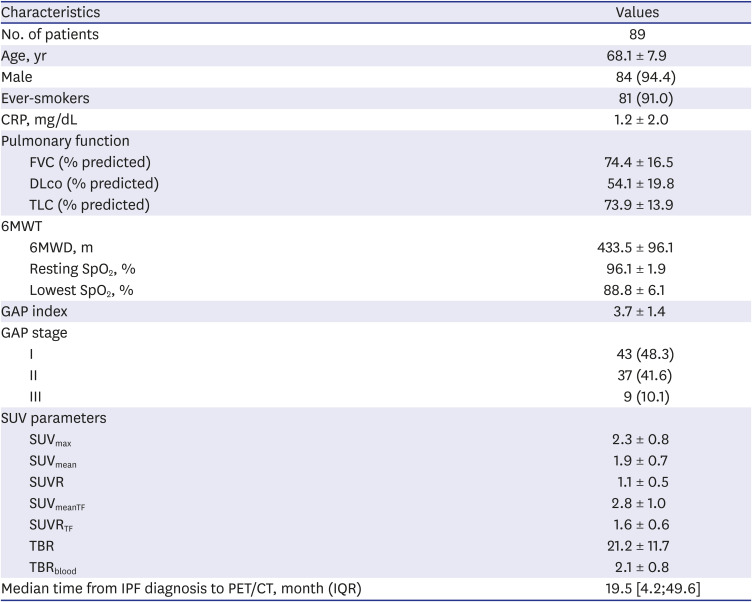
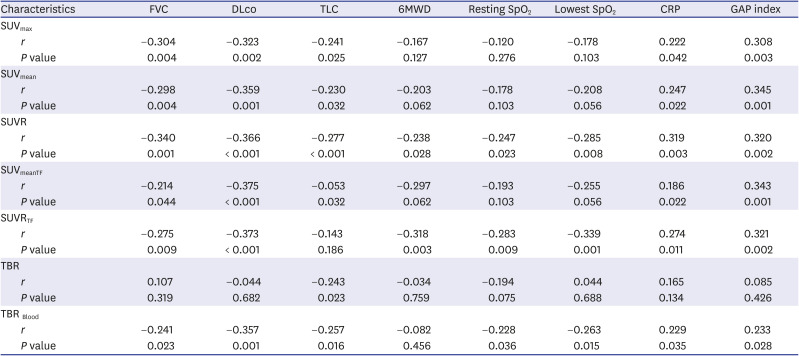
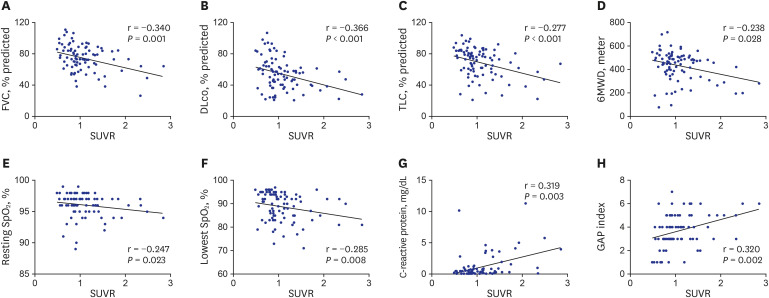




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download