Abstract
Purpose
The wrist, elbow, and axillae are recipient sites for vascularized lymph node transfer (VLNT) in upper extremity lymphedema. To the best of our knowledge, the possibility of the forearm as a recipient site for the VLNT has not been extensively investigated. We introduced a novel recipient site and surgical technique for VLNT in the distal upper extremity without a skin paddle.
Methods
Between January 2018 and February 2019, five consecutive patients underwent VLNT for upper extremity lymphedema. A vascularized supraclavicular lymph node was harvested and transferred to the mid-forearm of the lymphedematous limb. Radial artery, venae comitantes, and superficial vein were used as recipient vessels. Outcome was assessed by upper limb circumference and volume.
Results
All flaps survived without any donor-site morbidity. All patients reported symptom improvement. Mean circumference and volume at 3, 6, and 12 months after VLNT were reduced statistically significantly (p<0.05). Volume differential reduction was significant (p=0.005), showing an increasing tendency (p=0.050).
Lymphedema is a chronic condition of lymphatic impairment commonly caused by infection, surgery, or radiation therapy [1-4]. Inflammation caused by local immune insufficiency and abnormal lipid profiles also result in lymphatic impairment [5-9]. Disrupted lymphatic flow leads to fluid accumulation in the regional interstitium that could result in cellular proliferation, fibrosis, adipose tissue hypertrophy, and skin breakdown [2,10]. Owing to the chronic edematous conditions and inflammation, the postoperative rehabilitation and quality of life of lymphedema patients, approximately >250 million worldwide, are affected [2,5]. Despite the high incidence of lymphedema, it remains a challenge for reconstructive surgeons due to the lack of curative treatment options [1].
Numerous surgical options have been attempted as primary or adjunctive treatments of lymphedema, including excisional and physiologic methods [4]. Until now, vascularized lymph node transfer (VLNT) and lymphaticovenous anastomoses are the most promising techniques for chronic lymphedema [11-13]. Particularly, VLNT has become widely popular as treatment for lymphedema of the upper and lower extremities due to its convincing results and technical advantages compared to other treatments [14-16]. In this technique, the lymph nodes are transferred to lymphedematous extremities to improve lymph drainage [14].
Three recipient sites, namely, the wrist, elbow, and axillae, have been known to be available for VLNT in the upper extremity [17,18]. The recipient site is chosen according to the surgeon’s concept of transferred lymph node [18]. The surgeon who regards the flap as a pump prefers the distal sites where the pump is necessary. If the surgeon believes lymphangiogenesis or bridging is the main function, a more proximal location would be preferred. The obstruction location also could play an important role in selecting the recipient site. The lymph node flap may be placed near the obstruction site to maximize the effect of the pump or bridge.
Additionally, the cosmetic demand of the patient should be considered. Some patients want to achieve the best possible result through surgery, but other patients regard that the cosmesis is more important if the outcome of surgery would be acceptable. Unlike the axillary region, the elbow and wrist require a skin paddle for a tension-free inset [17]. The skin paddle may reduce the patients’ aesthetic satisfaction and may make the surgery more difficult.
To the best of our knowledge, the possibility of the forearm as a recipient site for the VLNT has not been extensively investigated. Thus, this study was designed to evaluate the forearm as a recipient site for the VLNT.
Following the approval by the Institutional Review Board of Pusan National University Hospital (No. H-1910-016-084), this study retrospectively reviewed five upper extremity lymphedema patients. The patients who can’t be managed by lymphovenous anastomosis underwent supraclavicular lymph node transfer from January 2015 to February 2019 at the Department of Plastic and Reconstructive Surgery, Pusan National University Hospital in Busan, Korea. Written informed consent was obtained from all patients.
Qualitative assessment and quantitative volumetric analysis were performed before lymph node transfer and at 3, 6, and 12 months postoperatively. Circumference measurements were obtained at the following three levels for each upper extremity: 10 cm above the elbow (AE), 10 cm below the elbow (BE), and the wrist. The measurements were repeated in triplicate at each follow-up. Upper extremity volume was calculated with circumference measurements as follows.
The volume differential, which is the excess volume of the affected lymphedematous upper extremity compared to the unaffected contralateral upper extremity, was calculated as follows: (volume of the affected lymphedematous upper extremity–volume of the unaffected contralateral upper extremity)/volume of the unaffected contralateral upper extremity. The volume differential reduction, which is the reduction of the excess volume of the affected lymphedematous upper extremity after lymph node transfer, was calculated as follows: (preoperative volume differential–postoperative volume differential)/preoperative volume differential.
The supraclavicular lymph node flaps were harvested without a skin paddle. The lymph nodes could be found in the triangular area surrounded by the sternocleidomastoid (SCM) muscle, clavicle, and external jugular vein (EJV). The incision was made from the lateral border of the SCM muscle to the EJV, which was 1 cm above the clavicle and approximately 5 cm long. After opening of the platysma muscle, which was found through the incision, the SCM muscle was exposed. Some branches of the supraclavicular nerves would be identified and preserved during the dissection. The omohyoid muscle was encountered under the medially retracted SCM muscle. The division of omohyoid muscle was followed by a careful dissection along the lateral border of the internal jugular vein. Lymphatic ducts were preserved or ligated carefully. The transverse cervical vessels (TCVs) are located deep under the omohyoid muscle. The thyrocervical trunk could be dissected if the transverse cervical artery (TCA) was small. At least two lymph nodes on the anterior scalene muscle were harvested with surrounding fat. Branches of the EJV, as superficial drainage system, and TCV, as deep drainage system and pedicle, should be included in the harvested lymph node. If an efferent lymphatic channel was identified during the harvest, it was also preserved in the lymph node for lymphaticolymphatic anastomosis.
For insetting, a 5-cm long incision was made on the medial aspect of the mid-forearm. Careful dissection was performed to preserve the superficial veins as a recipient vein. The subcutaneous fat tissue was resected to secure the space for the lymph node (Fig. 1). The space should be fit to the harvested supraclavicular lymph node. A larger space could result in hematoma or seroma, and a smaller space may compress the vascularized lymph node or wound problem. After resection of the adipose tissue, the intermuscular septum between the brachioradialis and flexor carpi radialis muscles was located to identify radial vessels to be used as recipient vessels.
The TCA of the lymph node was anastomosed to the radial artery with an end-to-side technique. End-to-end anastomoses of the veins were performed several times. The EJV branches were anastomosed to the superficial veins of the forearm to create a superficial drainage system. The TCV was anastomosed to the venae comitantes of the radial artery to make a deep drainage system. In one of the five cases, lymphaticolymphatic anastomosis was performed with the efferent lymphatic channel of the lymph node. After insetting, the incision was carefully sutured tension-free to prevent the compression of the transferred lymph node (Fig. 2).
All statistical analyses were performed with IBM SPSS Statistics ver. 24.0 (IBM Corp., Armonk, NY, USA). The mean differences of each group were calculated using Friedman test. A p-value of <0.05 was regarded as statistically significant and p<0.1 was considered to show a statistical tendency.
Vascularized supraclavicular lymph node transfer was performed in five upper extremities (Table 1). Three and two cases were located in the right and left extremities, respectively. All lymphedema cases were secondarily due to breast cancer surgery. Patients’ mean age was 59.4 years (range, 48–68 years). The mean duration of lymphedema was 5.7 years (1–20 years). The mean body mass index was 26.4 kg/m2 (range, 24.6–28.9 kg/m2).
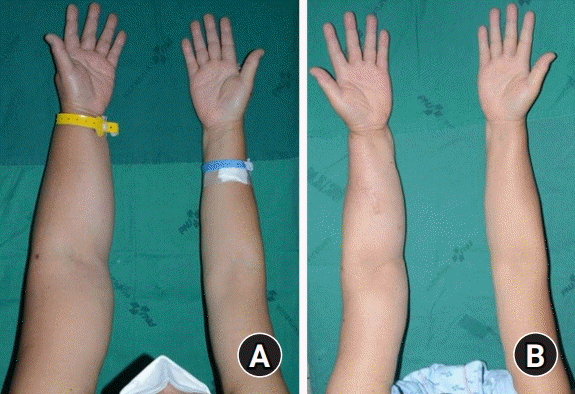
All flaps survived, and no donor-site morbidity was encountered (Fig. 3). The operative scars were acceptable and easily concealed under the sleeves. The survival and function of lymph node flap were verified with indocyanine green lymphangiography (Fig. 4).
All five patients reported an improvement of symptom postoperatively. The patients felt that the lymphedematous upper extremities were softer at first. Moreover, they reported that the affected limbs felt lighter and less painful compared with the preoperative state several months later.
The quantitative measurements are demonstrated in Table 2. The mean AE was reduced from 30.0 cm to 28.5 cm. The difference was statistically significant (p=0.004). The mean BE decreased from 26.5 cm to 26.0 cm, but the difference was not significant (p=0.611). The mean wrist circumference changed from 17.0 cm to 16.5 cm; although it was not significant, there was a statistical trend of reduction (p=0.061). The mean volume of the affected upper extremity was 1,916.4 cm3 preoperatively. The mean volume at 3, 6, and 12 months after lymph node transfer progressively decreased (1,833.3, 1,826.7, and 1,792.3 cm3, respectively) (Fig. 5), showing a statistically significant difference (p=0.015).
The mean volume differential of the affected limbs compared with that of the unaffected limbs was 40.5% (range, 19.6%–71.6%) preoperatively (Table 3). The mean volume differential consistently decreased at 3, 6, and 12 months after lymph node transfer (25.6%, 23.1%, and 20.2%, respectively). The reduction of volume differential was statistically significant (p=0.005).
The mean volume differential reduction increased at 3, 6, and 12 months after lymph node transfer (31.9%, 36.5%, and 45.6%, respectively) (Table 4). Although not statistically significant, there was a statistically increasing tendency of the volume differential reduction (p=0.050).
VLNT has become widely popular as treatment for lymphedema of the upper and lower extremities due to its convincing results and technical advantages compared to other options [14-16]. Additionally, liposuction or lymphovenous anastomosis for the treatment of lymphedema is mainly focused on the suprafascial compartment of the lymphedematous limb, but VLNT can absorb the lymph of both the suprafascial and subfascial compartments [19].
For selecting the recipient site, the following factors should be considered; mechanism of lymph node, scar release, and aesthetic concerns [20]. For VLNT in upper extremity lymphedema, the axilla, elbow, and wrist have been studied as recipient sites [17,20,21]. The axilla is often used in patients with symptomatic scar contracture, resulting from previous axillary lymph dissection or radiotherapy. Excisional release of scar tissue in the axilla can benefit the patient, although its dissection may be laborious and dangerous. Moreover, it can provide sufficient space to bury the transferred lymph node; thus, the aesthetic outcomes are superior to those of other recipient sites [20]. The elbow is a good option for patients with lymphedema in the upper arm, which is more severe than that of the forearm, or those with aesthetic concerns. Even though it is necessary to leave the skin paddle to obtain a tension-free inset, the cosmesis of the elbow is much better than that of the wrist [17]. The wrist is known to have the potential to achieve better outcomes [17,20]. The flap is typically placed on the dorsal side of the wrist; thus, positioning is very comfortable for microvascular surgery and postoperative care, in spite of its poorer cosmesis. To date, the consensus on the ideal recipient site has not been established.
In this situation, the forearm could be another excellent candidate for the recipient site in various aspects. The lymph node flap could be placed at the distal site of the upper extremity. Based on the theory of physiologic drainage as a pump, the surgeons may prefer the flap to be placed close to the area with the greatest necessity for its optimal function [18]. The location of the lymphatic obstruction also should be considered; thus, if the obstruction is distal, the surgeons prefer that the flap be implanted distally for physiologic bridging of the defect [18]. Furthermore, gravity is one of the important factors when selecting the recipient site, because it makes the drainage of the lymph of the proximal inset lymph nodes from the distal part of the extremities difficult [17]. In this aspect, the forearm, elbow, and wrist could be good options for achieving better surgical outcomes, while avoiding dangerous dissection and scar release, which commonly occur when the recipient site selected is the axilla.
For the patient with an aesthetic concern, the bulkiness and skin paddle of the flap could be a critical issue. The distal recipient sites, such as the elbow and wrist, inevitably require the skin paddle, and often even skin graft, to relieve the tension derived from the bulkiness of the flap for inset [17]. The skin paddle is a decisive factor that results in poor cosmetic outcomes for these recipient sites. Although the flap could be debulked and made inconspicuous in a few months, an initial unpleasant appearance and the fact that it requires secondary surgery are the reasons for the patients’ reluctance to undergo this procedure. Even for surgeons, the need for a skin paddle and skin grafting makes the surgery more difficult. However, the forearm as the recipient site does not require a skin paddle or skin graft for a tension-free flap inset. In the sufficient soft tissue at the medial aspect of the mid-forearm, the surgeons could secure enough space to bury the lymph node flap after undermining and excising the adipose tissue. Additionally, even the operative site is concealed under the sleeve in daily life.
The harvest of the lymph node without the skin paddle is advantageous in terms of aesthetics and anatomical structure preservation. To fix the skin paddle on the lymph node, the structures placed between the skin paddle and the lymph node are inevitably sacrificed, especially the supraclavicular nerves. Anterior chest wall numbness caused by iatrogenic supraclavicular nerve damage is a very common complication in the operation on the clavicular region [22]. Some patients even reported adverse effects on shoulder function, although the supraclavicular nerve is a sensory nerve [22]. Some branches of the supraclavicular nerve cross the clavicle according to the anatomical study; thus, the supraclavicular lymph node flap including the skin paddle, which is placed transversely above the clavicle, unavoidably contains more than one branch of the supraclavicular nerve [23]. However, because the nerve is a superficial nerve, the lymph node could be dissected avoiding the transection of branches, if the skin paddle is not necessary. Even if the nerve should be transected during the harvest, it could be easily anastomosed at the donor repair site. In fact, the branches of the supraclavicular nerve were identified and preserved during lymph node harvests in this study. To preserve the supraclavicular nerve and minimize donor morbidity, the selection of recipient site, which does not require a skin paddle, should be an important issue in lymph node transfer.
The fact that the location where the lymph node is transferred is a joint could be another point of controversy. All the traditional candidates for recipient sites, such as the axillae, elbow, and wrist, are joints. However, flap inset on the joint could cause movement discomfort and limitation of range of motion, because of its bulkiness. For the patients with a lymphedematous limb, this kind of inconvenience may lead to a more decline in their quality of life. In terms of postoperative care, the joints are not ideal options either. Joint motions could damage the anastomosed pedicles and lymphangiogenesis and result in poor wound healing. Contrarily, joint immobilization for postoperative care may stiffen the joint itself, which could not already move freely due to the lymphedema. To overcome these obstacles, the shaft of the limb, such as forearm, could be an alternative for the recipient site. The flap inset in the forearm does not influence the movement of nearby joints; thus, the patient does not experience any discomfort due to the transferred lymph node during activities of daily living. At the same time, the vascularized flap is isolated from the motion of joints postoperatively, which can cause adverse effects. It also helps in stabilizing the anastomosis of the vessels and in safely removing the lymph node.
In the forearm, the movement of the nearby joint, especially in the wrist, would not be harmful, but rather helpful to the transferred lymph node. To move the wrist, the forearm muscles should contract. This muscle contracture in the limited space, such as the forearm, leads to an increase in compartment pressure. The increasing pressure could act as a pump to squeeze the lymph node and lymphatic fluid in it. The pumping action may help in facilitating the lymphatic circulation in the lymphedematous limb by the transferred lymph node. This hypothesis should be validated, but it is worth to be reasonably considered.
Besides, the forearm provides several benefits for the surgical procedure of VLNT. First, the sufficient superficial venous system is distributed in the forearm, especially in the radial aspect [24]. The superficial venous communication has an important role in lymph node flap survival and promotion of lymphaticovenous anastomoses and lymphangiogenesis. The forearm can be easily positioned to expose the medial aspect of the mid-forearm, which is one of its advantages as a recipient site. This anatomical feature is comfortable for the surgeon when performing the microsurgical procedure and allows easy positioning during postoperative care to avoid pressure on the patient.
Through this study, the effectiveness of the forearm as a recipient site for the VLNT was also validated. The symptoms of lymphedematous limb were improved in all patients. Even in the quantitative analysis, the volume of the affected lymphedematous upper extremities was reduced from 1,916.4 cm3 to 1,792.3 cm3 after lymph node transfer at 12 months postoperatively. This statistically significant volume reduction proves that the lymph node could effectively function in the forearm. The volume of the affected limbs after lymph node transfer was also significantly reduced compared with the volume of the unaffected limbs and preoperative affected limb. All indicators showed that the forearm is among the excellent recipient sites for lymph node transfer.
In detail, circumference reduction was remarkable, especially in the upper arm and wrist. Even with the effort to secure its space in the recipient site, it seems that the volume of the lymph node itself acted as a confounder in the volumetric analysis of the forearm.
The small sample number and short follow-up period are the limitations of this study. Long-term follow-up in a large-scale study is necessary to confirm the effectiveness of the forearm as a recipient site for VLNT.
The forearm appears to be an excellent option as a recipient site in terms of its aesthetic and surgical benefits. VLNT in the forearm resulted in remarkable improvements in the circumferential and volume difference. Further study for larger series is needed to clarify the effect of forearm as a recipient site.
ACKNOWLEDGMENTS
This work was supported by clinical research grant from Pusan National University Hospital in 2020.
REFERENCES
1. Viitanen TP, Mäki MT, Seppänen MP, Suominen EA, Saaristo AM. Donor-site lymphatic function after microvascular lymph node transfer. Plast Reconstr Surg. 2012; 130:1246–53.

2. Najjar M, Lopez MM Jr, Ballestin A, et al. Reestablishment of lymphatic drainage after vascularized lymph node transfer in a rat model. Plast Reconstr Surg. 2018; 142:503e–508e.

3. Silva AK, Chang DW. Vascularized lymph node transfer and lymphovenous bypass: novel treatment strategies for symptomatic lymphedema. J Surg Oncol. 2016; 113:932–9.

4. Nicoli F, Orfaniotis G, Ciudad P, et al. Alternative vascular constructs of lymph node flap transfer. J Surg Oncol. 2018; 117:1144–7.

5. Yamamoto T, Iida T, Yoshimatsu H, Fuse Y, Hayashi A, Yamamoto N. Lymph flow restoration after tissue replantation and transfer: importance of lymph axiality and possibility of lymph flow reconstruction without lymph node transfer or lymphatic anastomosis. Plast Reconstr Surg. 2018; 142:796–804.
6. Kim KW, Song JH. Emerging roles of lymphatic vasculature in immunity. Immune Netw. 2017; 17:68–76.

7. Bernier-Latmani J, Petrova TV. Intestinal lymphatic vasculature: structure, mechanisms and functions. Nat Rev Gastroenterol Hepatol. 2017; 14:510–26.

8. Iwasaki D, Yamamoto Y, Murao N, Oyama A, Funayama E, Furukawa H. Establishment of an acquired lymphedema model in the mouse hindlimb: technical refinement and molecular characteristics. Plast Reconstr Surg. 2017; 139:67e–78e.
9. Ogata F, Fujiu K, Koshima I, Nagai R, Manabe I. Phenotypic modulation of smooth muscle cells in lymphoedema. Br J Dermatol. 2015; 172:1286–93.

11. Kwak MD, Machens HG. The lateral intercostal artery perforator as an alternative donor vessel for free vascularized lymph node transplantation. Arch Plast Surg. 2018; 45:275–9.

12. Koshima I, Inagawa K, Urushibara K, Moriguchi T. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000; 16:437–42.

14. Visconti G, Constantinescu T, Chen HC. The venous lymph-node flap. Microsurgery. 2016; 36:527–8.

15. Teven CM, Ooi AS, Inbal A, Chang DW. Implantable Doppler monitoring of buried free flaps during vascularized lymph node transfer. J Surg Oncol. 2017; 116:371–7.

16. Tourani SS, Taylor GI, Ashton MW. Vascularized lymph node transfer: a review of the current evidence. Plast Reconstr Surg. 2016; 137:985–93.
17. Cheng MH, Chen SC, Henry SL, Tan BK, Chia-Yu Lin M, Huang JJ. Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg. 2013; 131:1286–98.
18. Gould DJ, Mehrara BJ, Neligan P, Cheng MH, Patel KM. Lymph node transplantation for the treatment of lymphedema. J Surg Oncol. 2018; 118:736–42.

19. Chu SY, Wang SC, Chan WH, et al. Volumetric differences in the suprafascial and subfascial compartments of patients with secondary unilateral lower limb lymphedema. Plast Reconstr Surg. 2020; 145:1528–37.

20. Cheng MH, Chang DW, Patel KM. Principles and practice of lymphedema surgery. Edinburgh: Elsevier;2016.
21. Becker C, Assouad J, Riquet M, Hidden G. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006; 243:313–5.
22. You JM, Wu YS, Wang Y. Comparison of post-operative numbness and patient satisfaction using minimally invasive plate osteosynthesis or open plating for acute displaced clavicular shaft fractures. Int J Surg. 2018; 56:21–5.

Fig. 1.
Intraoperative finding of vascularized lymph node transfer in forearm. (A) Subcutaneous fat tissue was resected to secure space for the lymph node and radial vessels were identified. The amount of adipose tissue resected (B) should approximate to the volume of lymph node (C). (D) After inset, the tension-free repair was performed paying attention to compression of the lymph node.
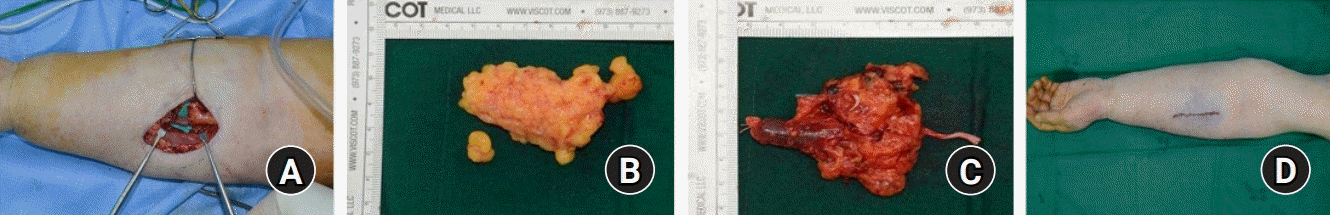
Fig. 2.
Illustration of mid-forearm after insetting the supraclavicular lymph node flaps. The transverse cervical artery of the lymph node was anastomosed to the radial artery and the transverse cervical vein was anastomosed to the venae comitantes of the radial artery.
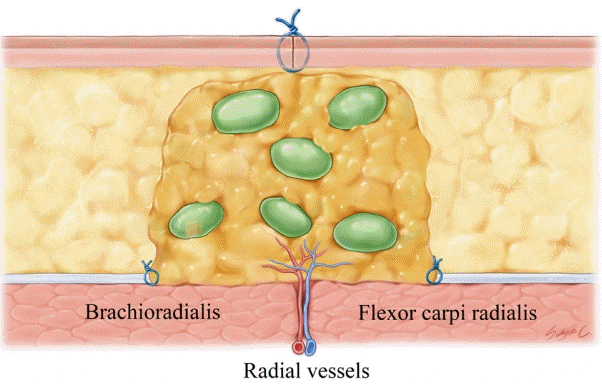
Fig. 4.
Preoperative (A) and 12 month-postoperative (B) indocyanine green lymphangiography of the patient 2.
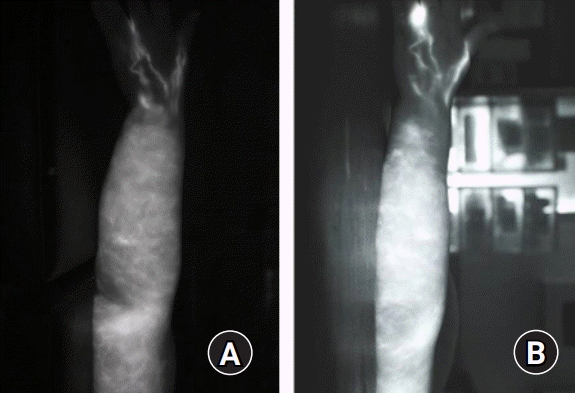
Fig. 5.
Change in the volume of the affected lymphedematous upper extremities at each postoperative follow-up period. Box-whisker plots show the mean (dot), median (horizontal line), interquartile range (box), and range (whiskers).
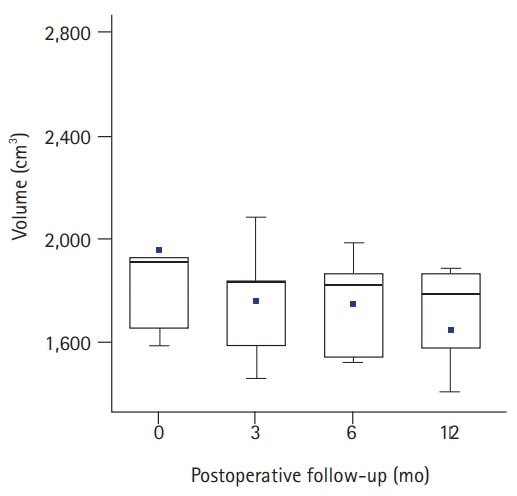
Table 1.
Demographics of the patients
| Variable |
Patient No. |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Age (yr) | 65 | 65 | 68 | 51 | 48 |
| Sex | Female | Female | Female | Female | Female |
| Body mass index (kg/m2) | 24.7 | 26.5 | 28.9 | 24.6 | 27.6 |
| Affected side | Left | Left | Right | Right | Right |
| Lymphedema duration (yr) | 1 | 20 | 4 | 2.5 | 1 |
| Stagea) | 4 | 4 | 4 | 4 | 3 |
Table 2.
The mean of measurements at each follow-up period
| Variable | Preoperative |
Postoperative (mo) |
p-value | ||
|---|---|---|---|---|---|
| 3 | 6 | 12 | |||
| Affected side | |||||
| Volume (cm3) | 1,916.4 (1,661.1–1,932.7) | 1,833.3 (1,594.6–1,839.2) | 1,826.7 (1,551.2–1,870.5) | 1,792.3 (1,584.0–1,870.5) | 0.015** |
| AE (cm) | 30.0 (30.0–31.5) | 29.0 (28.5–31.0) | 28.5 (28.5–30.0) | 28.5 (28.0–30.0) | 0.004** |
| BE (cm) | 26.5 (24.5–27.0) | 26.0 (24.0–27.0) | 27.0 (24.0–27.0) | 26.0 (24.0–27.0) | 0.611 |
| Wrist (cm) | 17.0 (16.5–19.0) | 16.5 (16.0–16.5) | 16.5 (16.5–16.5) | 16.5 (16.0–16.5) | 0.061* |
| Unaffected side | |||||
| Volume (cm3) | 1,333.2 (1,293.8–1,476.9) | 1,332.9 (1,302.9–1,476.9) | 1,393.9 (1,313.2–1,499.4) | 1,393.9 (1,302.9–1,520.7) | 0.233 |
| AE (cm) | 27.0 (25.5–27.5) | 27.0 (26.0–27.5) | 27.0 (26.5–27.0) | 26.5 (26.0–27.0) | 0.701 |
| BE (cm) | 21.5 (21.0–23.0) | 22.0 (21.5–23.0) | 22.5 (21.5–23.5) | 22.5 (21.5–24.0) | 0.172 |
| Wrist (cm) | 16.0 (15.5–16.0) | 15.0 (15.0–16.0) | 15.5 (15.5–16.5) | 15.5 (15.5–16.0) | 0.510 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download