Abstract
For years, neoadjuvant chemotherapy for locally advanced pancreatic cancer is being investigated and radical surgical resection with laparoscopic approach is getting up to speed. Pathological complete remission is known as a predictive marker for a good prognosis for various carcinomas. Although there are a few case reports about pathological complete remission, there has been no case report of pathological complete remission resulted from successful extensive resection by laparoscopic surgery after a neoadjuvant modified FOLFIRINOX chemotherapy. A 68-year-old male patient was admitted due to a palpable abdominal mass which turned out to be 16-cm-sized huge locally advanced left-sided pancreatic cancer with possible stomach, left adrenal gland, left kidney, and colon invasion. After administration of 10th modified FOLFIRINOX chemotherapy, the tumor had decreased and he underwent laparoscopic radical distal pancreatectomy with splenectomy, left adrenalectomy, wedge resection of stomach, and segmental resection of transverse colon. Although patient had a postoperative micro-abscess around the colon anastomosis site, he was successfully managed with conservative treatment and discharged on 12 days postoperatively. The final pathology reported complete tumor regression. We hereby emphasize the oncologic significance of neoadjuvant chemotherapy in huge left-sided pancreatic cancer and the potential role of laparoscopic conversion surgery.
Pancreatic cancer is a cancer with a poor prognosis. Its 5-year survival rate is less than 5% [1]. Although surgical resection is the most effective treatment, only 15% to 20% of patients could be operated at the time of diagnosis because most patients are found to be in an advanced stage of the cancer [1]. Thus, neoadjuvant chemotherapy, which enables surgery for advanced pancreatic cancer that could not be operated at time of diagnosis, is gaining prominence in pancreatic cancer. For years, neoadjuvant chemotherapy for locally advanced pancreatic cancer is being investigated and radical surgical resection with laparoscopic method is getting up to speed.
Locally advanced pancreatic cancer is mostly associated with major vascular involvement [2]. In particular, when the superior mesenteric artery or the celiac trunk is surrounded by a tumor, or when the superior mesenteric vein/superior mesenteric vein-portal vein confluence is blocked, it can be defined as a locally advanced or un-resectable case [2]. For such cases, neoadjuvant chemotherapy is recommended. FOLFIRINOX (fluorouracil, oxaliplatin, leucovorin, irinotecan) regimen has been established itself as a standard treatment for locally advanced pancreatic cancer [3-5].
In the past, for a locally advanced pancreatic cancer, it was natural to proceed with open surgery because surgical access was difficult and extensive combined resection of surrounding organs, massive bleeding, and long operation time were expected. However, advances in laparoscopic technology have enabled us to see the potential of extensive laparoscopic resection for well selected locally advanced pancreatic cancer [6].
In various carcinomas, pathological complete remission is known as a predictive marker for a good prognosis [7]. Moreover, confirming a complete response of the tumor after neoadjuvant chemotherapy is expected to predict a patient’s long-term survival [7]. Much research is needed on whether complete remission means a good survival even for pancreatic cancer with a poor prognosis. Although few case reports have been reported [8,9], there has been no case reports of pathological complete remission due to successful extensive resection by laparoscopic surgery after a neoadjuvant modified FOLFIRINOX chemotherapy.
We report a case of pathological complete remission of locally advanced pancreatic cancer successfully managed by laparoscopic radical resection following a neoadjuvant modified FOLFIRINOX chemotherapy.
A 68-year-old male patient had visited our outpatient clinic due to a palpable abdominal mass presented from 3 months ago. The patient had diabetes mellitus, chronic obstructive pulmonary disease, and former prostate cancer treated with proton therapy 13 years ago. Initial radiological evaluations showed a 16-cm-sized large solid mass in the pancreas body and tail with splenic hilum lymph node enlargements, splenic vessel invasion, and possible tumor abutment to the stomach, the left adrenal gland, the left kidney, and the colon. No other major vessel involvement was noted (Fig. 1). Endoscopic ultrasonography-guided biopsy confirmed carcinoma with acinar pattern and extensive necrosis, consistent with acinar cell carcinoma. There was no distant metastasis found in chest computed tomography (CT) or positron emission tomography computed tomography (PET-CT).
A diagnostic laparoscopy was done on November 21st, 2019. Intraoperative findings were compatible with preoperative image. It was observed that there was diffuse invasion of the tumor to the mid-body of stomach with presumed adjacent organ invasions. The tumor even had extended to pancreatic head lesions, requiring total pancreatectomy for potential curative resection. Considering potential postoperative co-morbidity related to total pancreatectomy with combined multi-visceral resection, and aggressive tumor biology, an attempt for curative resection was aborted. Under the plan for neoadjuvant chemotherapy, the patient was discharged two days after postoperative care.
The first chemotherapy was started on January 23rd, 2020. Modified FOLFIRINOX [4] (oxaliplatin, 85 mg/m2; leucovorin, 400 mg/m2; irinotecan, 150 mg/m2; and continuous fluorouracil, 2,400 mg/m2; without bolus fluorouracil) with 20% dose reduction regimen was used at 2 week intervals. The patient well-tolerated the therapy. He did not suffer severe chemotherapy-related complications other than mild nausea. After 10th doses, follow-up CT scan showed largely decreased tumor size from 16 cm to 10 cm without celiac or superior mesenteric artery invasion. No peritoneal seeding or distant metastasis was noted (Fig. 2).
On July 10th, 2020, laparoscopic radical distal pancreatectomy was attempted. There was a large cystic tumor occupying pancreas tail, still abutting to the stomach posterior wall. Pancreas head lesion seemed to be preserved from the tumor. The pancreatic neck portion was safely dissected and divided. No definite tumor invasion around the celiac axis or the superior mesenteric artery was noted. Soft tissues around the celiac axis were dissected and both the splenic artery and the vein were controlled. Stomach posterior wall was sleeved-resected with endoscopic gastrointestinal anastomosis stapler and re-enforced with knotless suture. Some parts of the splenic flexure of colon were invaded. Combined en bloc resection of colonic segment was performed. For tangential margin clearance, dissection of retroperitoneal soft tissues around the left kidney and left adrenalectomy were performed (Fig. 3). After bleeding control, two open silastic drains were inserted to be placed at the splenectomy site and the pancreatectomy site each. Total operation time was 437 minutes. Blood loss was 600 mL without intraoperative transfusion. The patient was awoken and sent to the post-anesthesia care unit. Finally, he was sent to the general ward.
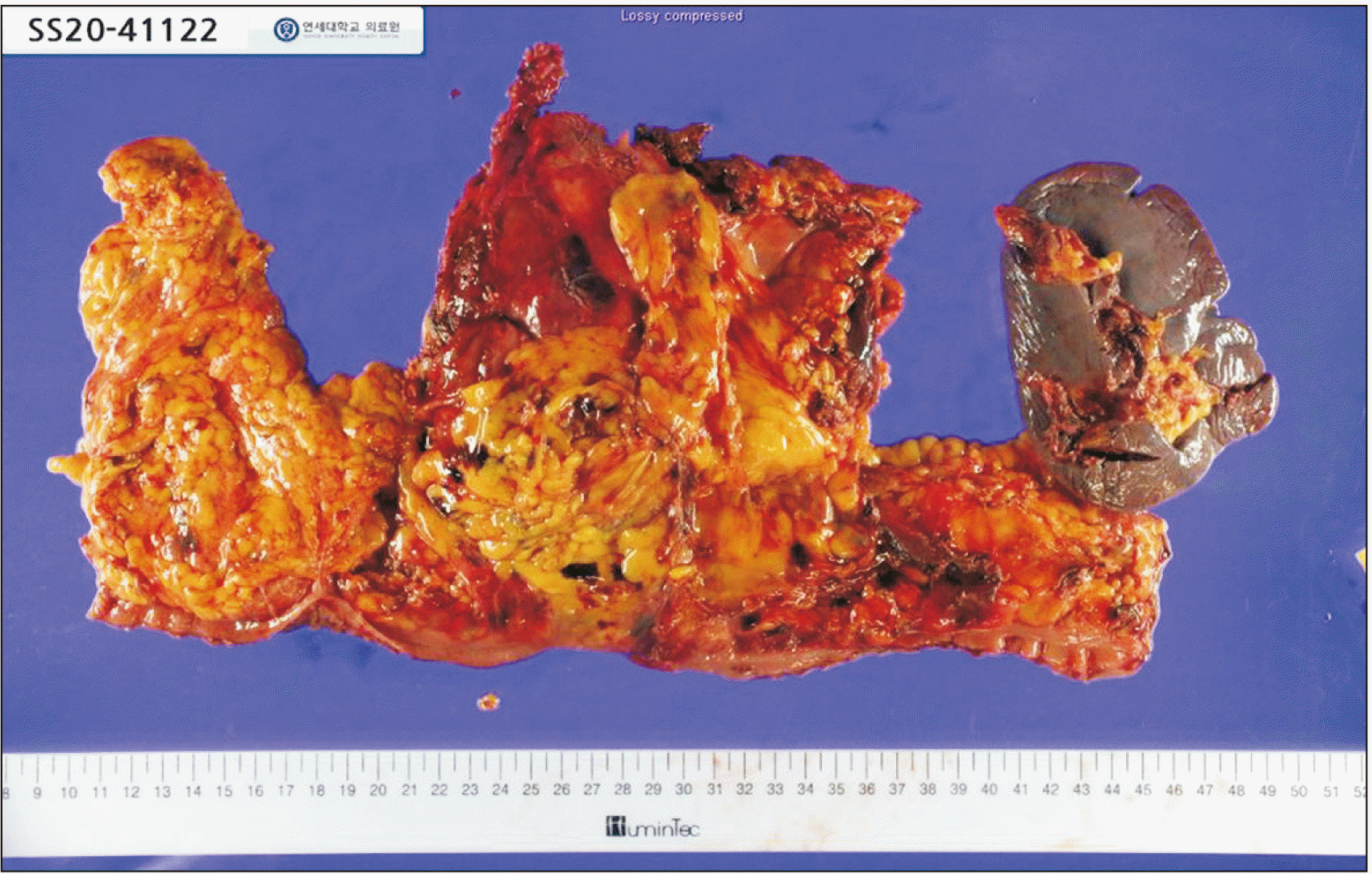
Postoperative days (POD) were uneventful until POD #5 when the open drain was removed and his fever spiked with mild leukocytosis observed. Abdominopelvic CT scan showed possible local peritonitis or ischemia of colon anastomosis with scarce fluid collection. However, conservative management with broad-spectrum intravenous antibiotics, transient nil per os, and nutritional support was sufficient. Finally, he was discharged at 12 days postoperatively. The final pathologic report revealed complete regression of the tumor without residual carcinoma, showing cystic degeneration with necrosis (Fig. 4). He is now just followed up without chemotherapy. Up to date, there is no evidence of tumor recurrence.
Preoperative chemotherapy for pancreatic cancer has become popular over the years. Neoadjuvant chemotherapy has its power in that it can increase the resectability by downsizing the tumor, increase R0 resection rate, cure micro-metastasis, and pre-select patients with good response to chemotherapy.
Many randomized clinical trial and meta-analysis have shown the superiority of a neoadjuvant therapy in borderline resectable pancreas cancer [10,11]. Several randomized clinical trials are undergoing using modified FOLFIRINOX. Several retrospective studies have already shown that preoperative modified FOLFIRINOX can lead to improved resectability, downstaged tumor, and increased disease-free survival and overall survival [3-5].
Conversion surgery in unresectable pancreatic cancer after treatment with chemotherapy has empowered itself from many cases [5,12]. Neoadjuvant chemotherapy including modified FOLFIRINOX can improve the resectability and R0 resection rate of locally advanced pancreatic cancer [5,13].
Meanwhile, laparoscopic distal pancreatectomy for left sided pancreas cancer is known to have results comparable (or even feasible) to a conventional open distal pancreatectomy. Laparoscopic approach can result in less intraoperative blood loss, intraoperative transfusion, time to first flatus, time to start diet, length of hospital stay and perioperative complications [14,15]. Although controversy still exists, laparoscopic treatment could be endorsed within effective treatment modality for a left sided pancreatic cancer [16].
Laparoscopic surgery is often performed for an advanced left side pancreatic cancer. So far, there has been no report about a randomized control trial-determine whether a laparoscopic surgery has oncological significance in advanced left side pancreatic cancer. However, with the widespread use of FOLFIRINOX, laparoscopic surgery is expected to increase in patients with a complete response after neoadjuvant FOLFIRINOX as a locally advanced pancreatic cancer in clinical practice. Therefore, careful experience and research on these patients are needed.
Our institution has been performing laparoscopic radical distal pancreatectomy for left sided pancreas cancer for years for well-selected patients. Outcomes of a laparoscopic radical distal pancreatectomy were no inferior to those of an open surgery [17,18]. Moreover, nowadays locally advanced pancreatic cancer can be properly managed with neoadjuvant therapy and subsequent operation in well-selected patients [8].
There are not so many case reports that illustrate locally advanced pancreatic cancer that totally resolves after a preoperative chemotherapy. Luu et al. [9] have demonstrated a patient with pancreatic head cancer involving portal vein that has been treated with five cycles of FOLFIRINOX. The final pathologic report was complete resolution. However the pathologic complete response did not designate an absolute cure. Some say they recur in up to 40%, which urges us to follow up patients more carefully and perform serial adjuvant chemotherapy as much as possible [19]. Also, pancreatic acinar cell carcinoma is a subtype that is rarely known for its chemoresponsiveness and surgical outcomes [20]. We should bear in mind that pancreatic acinar cell carcinoma does not represent most pancreatic cancer, which is a limitation when generalizing results of this study.
We hereby report a case of pathological complete remission of locally advanced pancreatic cancer successfully managed by a laparoscopic radical distal pancreatectosplenectomy with a combined gastric wedge resection and a segmental resection of the colon after he was administrated with the neoadjuvant modified FOLFIRINOX chemotherapy. Most pancreatic cancer patients might be disappointed because they are initially found in an advanced state with cure seeming difficult. However, potent neoadjuvant chemotherapy and subsequent safe curative pancreatectomy based on the patient’s eagerness for cure can brighten the future of pancreatic cancer.
REFERENCES
1. Li D, Xie K, Wolff R, Abbruzzese JL. 2004; Pancreatic cancer. Lancet. 363:1049–1057. DOI: 10.1016/S0140-6736(04)15841-8. PMID: 21620466. PMCID: PMC3062508.

2. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. 2006; Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 13:1035–1046. DOI: 10.1245/ASO.2006.08.011. PMID: 16865597.

3. Barenboim A, Lahat G, Geva R, Nachmany I, Nakache R, Goykhman Y, et al. 2018; Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: an intention to treat analysis. Eur J Surg Oncol. 44:1619–1623. DOI: 10.1016/j.ejso.2018.07.057. PMID: 30146251.

4. Blazer M, Wu C, Goldberg RM, Phillips G, Schmidt C, Muscarella P, et al. 2015; Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol. 22:1153–1159. DOI: 10.1245/s10434-014-4225-1. PMID: 25358667. PMCID: PMC4373613.

5. Nanda RH, El-Rayes B, Maithel SK, Landry J. 2015; Neoadjuvant modified FOLFIRINOX and chemoradiation therapy for locally advanced pancreatic cancer improves resectability. J Surg Oncol. 111:1028–1034. DOI: 10.1002/jso.23921. PMID: 26073887.

6. Hong SS, Hwang HK, Lee WJ, Kang CM. 2020; Feasibility and safety of laparoscopic radical distal pancreatosplenectomy with adrenalectomy in advanced pancreatic cancer. Ann Surg Oncol. 27:5235–5236. DOI: 10.1245/s10434-020-08670-9. PMID: 32474822.

7. Lorenzen S, Thuss-Patience P, Al-Batran SE, Lordick F, Haller B, Schuster T, et al. 2013; Impact of pathologic complete response on disease-free survival in patients with esophagogastric adenocarcinoma receiving preoperative docetaxel-based chemotherapy. Ann Oncol. 24:2068–2073. DOI: 10.1093/annonc/mdt141. PMID: 23592699.

8. Valeri S, Borzomati D, Nappo G, Perrone G, Santini D, Coppola R. 2014; Complete pathological response after FOLFIRINOX for locally advanced pancreatic cancer. The beginning of a new era? Case report and review of the literature. Pancreatology. 14:425–430. DOI: 10.1016/j.pan.2014.07.002. PMID: 25278312.

9. Luu AM, Herzog T, Hoehn P, Reinacher-Schick A, Munding J, Uhl W, et al. 2018; FOLFIRINOX treatment leading to pathologic complete response of a locally advanced pancreatic cancer. J Gastrointest Oncol. 9:E9–E12. DOI: 10.21037/jgo.2018.01.07. PMID: 29755782. PMCID: PMC5934161.

10. Cloyd JM, Heh V, Pawlik TM, Ejaz A, Dillhoff M, Tsung A, et al. 2020; Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med. 9:1129. DOI: 10.3390/jcm9041129. PMID: 32326559. PMCID: PMC7231310.

11. Versteijne E, Vogel JA, Besselink MG, Busch ORC, Wilmink JW, Daams JG, et al. 2018; Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 105:946–958. DOI: 10.1002/bjs.10870. PMID: 29708592. PMCID: PMC6033157.

12. Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, et al. 2013; Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 20:590–600. DOI: 10.1007/s00534-013-0616-0. PMID: 23660962.
13. Yoshida K, Iwashita T, Uemura S, Maruta A, Okuno M, Ando N, et al. 2017; A multicenter prospective phase II study of first-line modified FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget. 8:111346–111355. DOI: 10.18632/oncotarget.22795. PMID: 29340058. PMCID: PMC5762326.

14. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, et al. 2015; A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg. 220:177–185. DOI: 10.1016/j.jamcollsurg.2014.10.014. PMID: 25529901.

15. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. 2019; Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. 269:2–9. DOI: 10.1097/SLA.0000000000002979. PMID: 30080726.
16. Sui CJ, Li B, Yang JM, Wang SJ, Zhou YM. 2012; Laparoscopic versus open distal pancreatectomy: a meta-analysis. Asian J Surg. 35:1–8. DOI: 10.1016/j.asjsur.2012.04.001. PMID: 22726557.

17. Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. 2014; Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 28:2848–2855. DOI: 10.1007/s00464-014-3537-3.
18. Lee SH, Hwang HK, Kang CM, Lee WJ. 2017; The Yonsei criteria as a clinically detectable parameter for excellent prognosis in resected left-sided pancreatic cancer: outcomes of a propensity score-matched analysis. Surg Endosc. 31:4656–4664. DOI: 10.1007/s00464-017-5529-6. PMID: 28389802.

19. Lee SH, Kang CM, Kim H, Hwang HK, Song SY, Seong J, et al. 2015; Pathological complete remission of pancreatic cancer following neoadjuvant chemoradiation therapy; not the end of battles. Medicine (Baltimore). 94:e2168. DOI: 10.1097/MD.0000000000002168. PMID: 26717359. PMCID: PMC5291600.

20. Chaudhary P. 2015; Acinar cell carcinoma of the pancreas: a literature review and update. Indian J Surg. 77:226–231. DOI: 10.1007/s12262-014-1049-y. PMID: 26246707. PMCID: PMC4522262.

Fig. 1
(A, B) Initial abdominopelvic computed tomography showing a 16-cm sized mass in the pancreas body and tail abutting to stomach, left adrenal gland, left kidney, and colon with splenic vessel invasion.
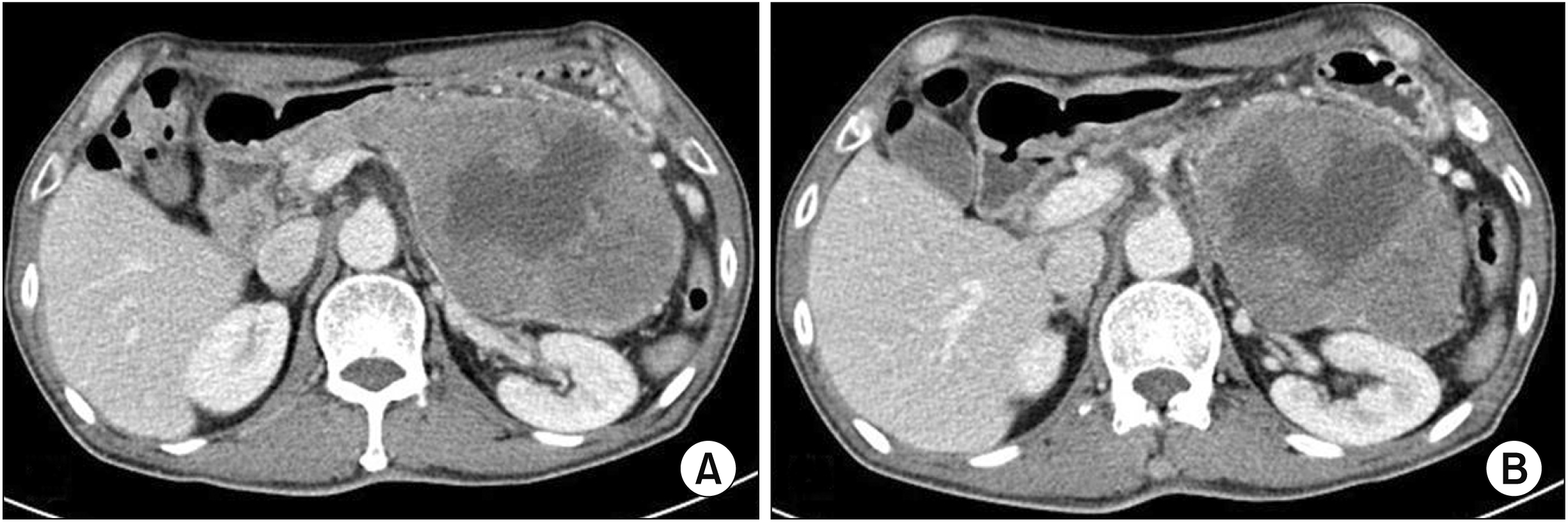
Fig. 2
(A, B) Abdominopelvic computed tomography after administration of the 10th doses of modified FOLFIRINOX, showing decreased size and solidity of mass (arrow).
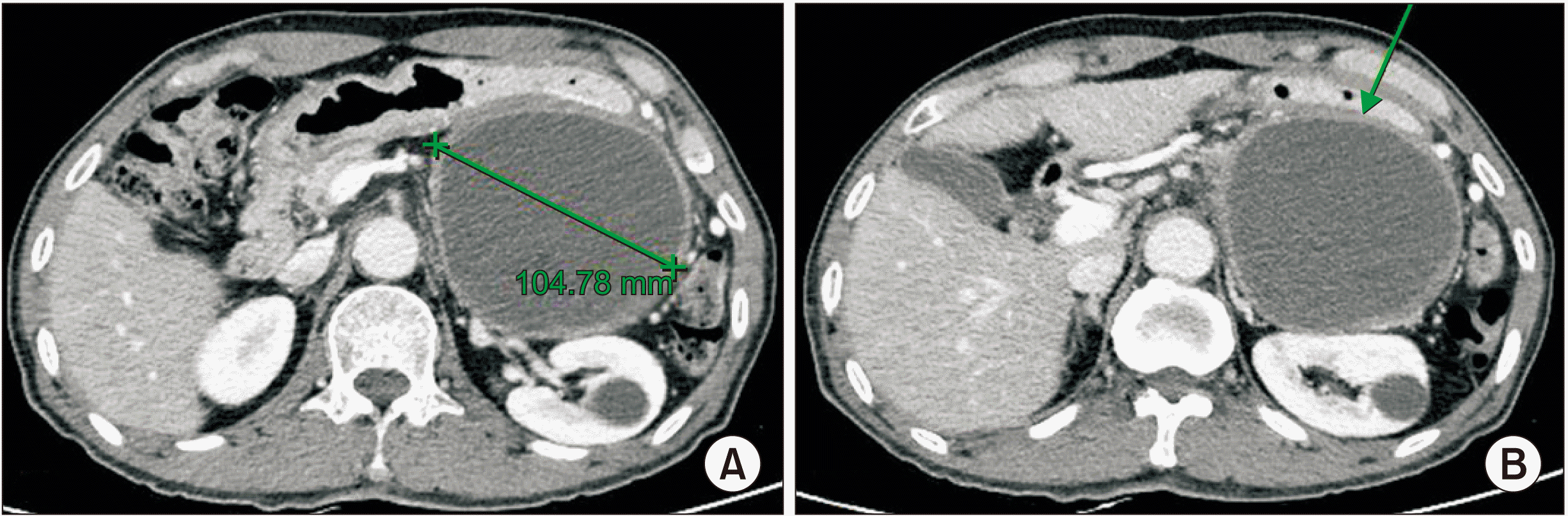
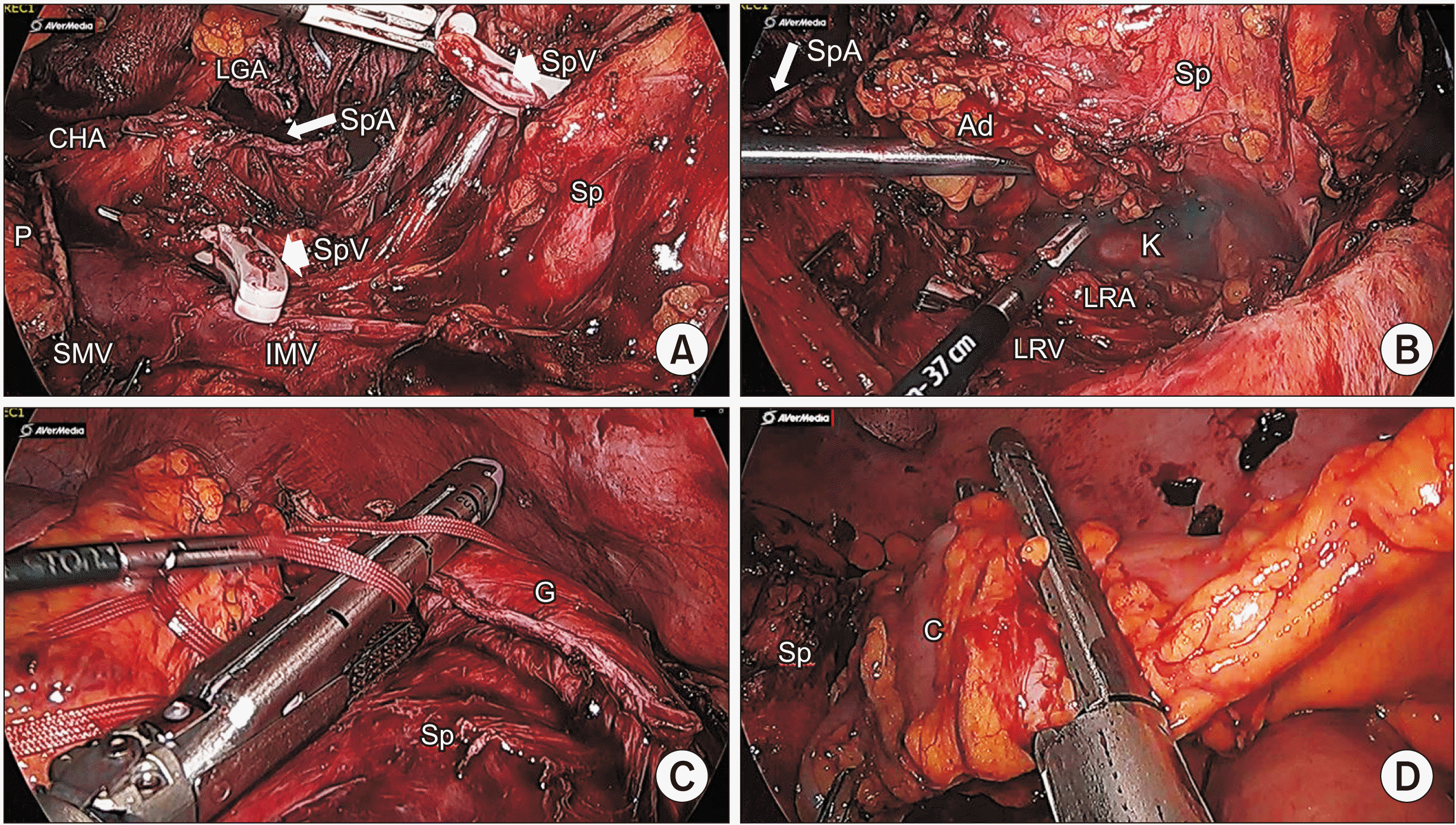
Fig. 3
(A) SpA (long white arrow) and SpV (short white arrows) were ligated and celiac artery dissection was done. (B) Left adrenal gland was resected and left pararenal fat layer were dissected. Note LRA and LRV, as well as resected spelnic artery (long white arrow). (C) Laparoscopic wedge resection of the stomach was performed with endoscopic gastrointestinal anasomosis (GIA) stapler. (D) Segmental resection of the affected colon was done with GIA stapler. CHA, common hepatic artery; LGA, left gastric artery; SpA, splenic artery; SpV, splenic vein; SMV, superior mesenteric vein; IMV, inferior mesenteric vein; Sp, spleen; P, pancreas; Ad, adrenal gland; LRA, left renal artery; LRV, left renal vein; K, kidney; G, stomach; C, colon.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download