1. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994; 117(4):429–441. PMID:
8154523.

2. Park SJ, Choi NK, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed retinal vein occlusion in Korea, 2008 through 2011: preponderance of women and the impact of aging. Ophthalmology. 2014; 121(6):1274–1280. PMID:
24491641.
3. Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010; 117(2):313–319.e1. PMID:
20022117.

4. Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008; 38(12):904–910. PMID:
19120547.

5. Youm DJ, Oh HS, Yu HG, Song SJ. The prevalence of vitreoretinal diseases in a screened Korean population 50 years and older. J Korean Ophthalmol Soc. 2009; 50(11):1645–1651.

6. Deobhakta A, Chang LK. Inflammation in retinal vein occlusion. Int J Inflamm. 2013; 2013:438412.

7. Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Surv Ophthalmol. 2011; 56(4):281–299. PMID:
21601903.

8. Hayreh SS, Podhajsky PA, Zimmerman MB. Central and hemicentral retinal vein occlusion: role of anti-platelet aggregation agents and anticoagulants. Ophthalmology. 2011; 118(8):1603–1611. PMID:
21704382.
9. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013; 33(5):901–910. PMID:
23609064.
10. Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008; 33(2):111–131. PMID:
18293182.

11. Wu CY, Riangwiwat T, Limpruttidham N, Rattanawong P, Rosen RB, Deobhakta A. Association of retinal vein occlusion with cardiovascular events and mortality: a systematic review and meta-analysis. Retina. 2019; 39(9):1635–1645. PMID:
30829987.
12. Lee JY, Yoon YH, Kim HK, Yoon HS, Kang SW, Kim JG, et al. Baseline characteristics and risk factors of retinal vein occlusion: a study by the Korean RVO Study Group. J Korean Med Sci. 2013; 28(1):136–144. PMID:
23341724.

13. Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009; 4(12):e8158. PMID:
19997642.

14. Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. 'United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2003; 42(6 ):Suppl 5. A5–A7. S1–230. PMID:
14655179.
15. Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011; 80(12):1258–1270. PMID:
21993585.

16. Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005; 365(9456):331–340. PMID:
15664230.
17. Jin DC, Han JS. Renal replacement therapy in Korea, 2012. Kidney Res Clin Pract. 2014; 33(1):9–18. PMID:
26877945.

18. Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014; 29(1):29–40. PMID:
24132242.

19. Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001; 131(1):61–77. PMID:
11162981.

20. Moody WE, Edwards NC, Chue CD, Ferro CJ, Townend JN. Arterial disease in chronic kidney disease. Heart. 2013; 99(6):365–372. PMID:
23118349.

21. Wong TY, Larsen EK, Klein R, Mitchell P, Couper DJ, Klein BE, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology. 2005; 112(4):540–547. PMID:
15808241.
22. Arakawa S, Yasuda M, Nagata M, Ninomiya T, Hirakawa Y, Doi Y, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general Japanese population: the Hisayama Study. Invest Ophthalmol Vis Sci. 2011; 52(8):5905–5909. PMID:
21693603.

23. Chang YS, Weng SF, Chang C, Wang JJ, Tseng SH, Wang JY, et al. Risk of retinal vein occlusion following end-stage renal disease. Medicine (Baltimore). 2016; 95(16):e3474. PMID:
27100450.

24. Chen SN, Yang TC, Lin JT, Lian IB. End stage renal disease as a potential risk factor for retinal vein occlusion. Medicine (Baltimore). 2015; 94(47):e1960. PMID:
26632690.

25. Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006; 124(5):726–732. PMID:
16682596.
26. Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008; 126(4):513–518. PMID:
18413521.
27. Lee KS, Nam KH, Kim DW, Kang EC, Koh HJ. Risk of retinal vein occlusion in patients with end-stage renal disease: a 12-year, retrospective, nationwide cohort study in South Korea. Invest Ophthalmol Vis Sci. 2018; 59(1):39–44. PMID:
29302692.

28. Alpert MA, Van Stone J, Twardowski ZJ, Ruder MA, Whiting RB, Kelly DL, et al. Comparative cardiac effects of hemodialysis and continuous ambulatory peritoneal dialysis. Clin Cardiol. 1986; 9(2):52–60. PMID:
3948441.

29. Yong K, Dogra G, Boudville N, Lim W. Increased inflammatory response in association with the initiation of hemodialysis compared with peritoneal dialysis in a prospective study of end-stage kidney disease patients. Perit Dial Int. 2018; 38(1):18–23. PMID:
29097485.

30. Kwon SK, Han JH, Kim HY, Kang G, Kang M, Kim YJ, et al. The incidences and characteristics of various cancers in patients on dialysis: a Korean nationwide study. J Korean Med Sci. 2019; 34(25):e176. PMID:
31243935.

31. Min J, Kwon SK, Jeong HW, Han JH, Kim YJ, Kang M, et al. End-stage renal disease and risk of active tuberculosis: a nationwide population-based cohort study. J Korean Med Sci. 2018; 33(53):e341. PMID:
30595682.

32. Izzedine H, Bodaghi B, Launay-Vacher V, Deray G. Eye and kidney: from clinical findings to genetic explanations. J Am Soc Nephrol. 2003; 14(2):516–529. PMID:
12538754.

33. Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol. 2005; 16(5):1392–1403. PMID:
15800116.

34. Deva R, Alias MA, Colville D, Tow FK, Ooi QL, Chew S, et al. Vision-threatening retinal abnormalities in chronic kidney disease stages 3 to 5. Clin J Am Soc Nephrol. 2011; 6(8):1866–1871. PMID:
21784818.

35. Lim LS, Cheung CY, Sabanayagam C, Lim SC, Tai ES, Huang L, et al. Structural changes in the retinal microvasculature and renal function. Invest Ophthalmol Vis Sci. 2013; 54(4):2970–2976. PMID:
23572105.

36. Chang JH, Sung JY, Ahn SY, Ko KP, Ro H, Jung JY, et al. Hemodialysis leads to better survival in patients with diabetes or high comorbidity, compared to peritoneal dialysis. Tohoku J Exp Med. 2013; 229(4):271–277. PMID:
23603422.
37. Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. 2017; 2017:3081856. PMID:
29138677.

38. Liakopoulos V, Roumeliotis S, Gorny X, Eleftheriadis T, Mertens PR. Oxidative stress in patients undergoing peritoneal dialysis: a current review of the literature. Oxid Med Cell Longev. 2017; 2017:3494867. PMID:
29750088.

39. Cheung N, Klein R, Wang JJ, Cotch MF, Islam AF, Klein BE, et al. Traditional and novel cardiovascular risk factors for retinal vein occlusion: the multiethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. 2008; 49(10):4297–4302. PMID:
18539932.

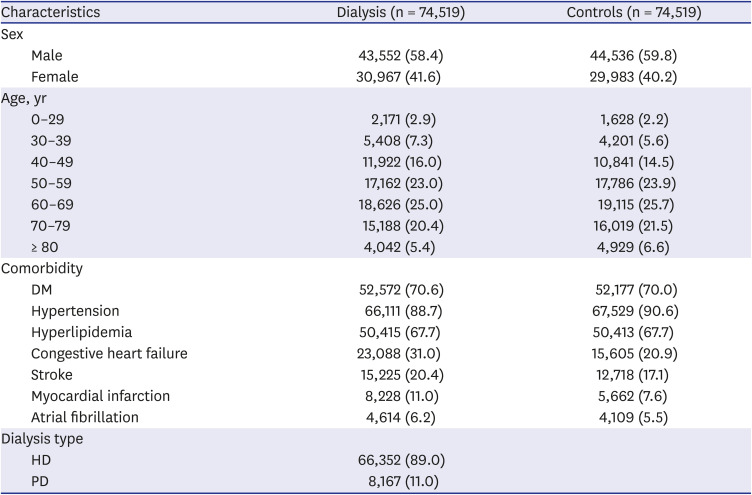
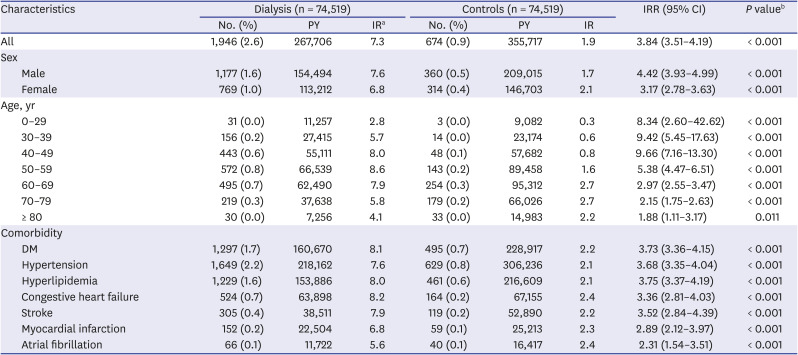
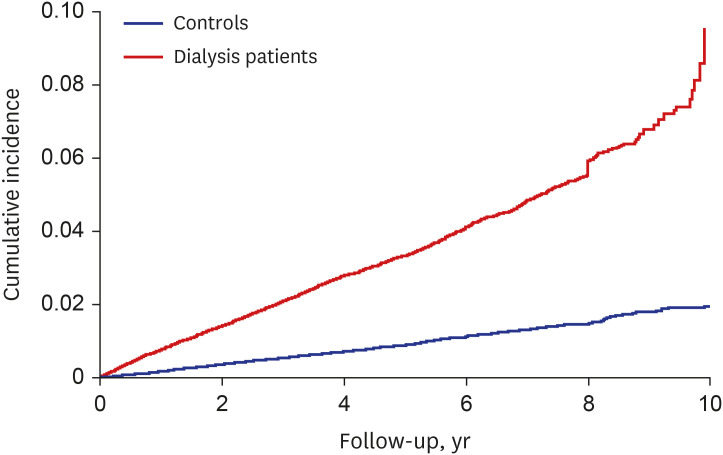
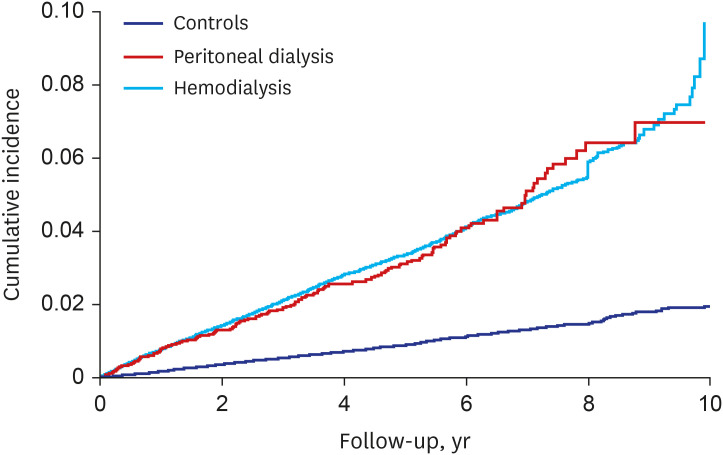




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download