1. Holmberg MJ, Ross CE, Fitzmaurice GM, Chan PS, Duval-Arnould J, Grossestreuer AV, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019; 12(7):e005580. PMID:
31545574.

2. Hawkes C, Booth S, Ji C, Brace-McDonnell SJ, Whittington A, Mapstone J, et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation. 2017; 110:133–140. PMID:
27865775.

3. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020; 141(9):e139–e596. PMID:
31992061.

4. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, et al. European resuscitation council and European society of intensive care medicine guidelines for post-resuscitation care 2015: section 5 of the European resuscitation council guidelines for resuscitation 2015. Resuscitation. 2015; 95:202–222. PMID:
26477702.
5. Panchal AR, Bartos JA, Cabanas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020; 142(16_):suppl_2. S366–S468. PMID:
33081529.

6. Kim YM, Park KN, Choi SP, Lee BK, Park K, Kim J, et al. Part 4. post-cardiac arrest care: 2015 Korean guidelines for cardiopulmonary resuscitation. Clin Exp Emerg Med. 2016; 3(Suppl):S27–S38. PMID:
27752644.

7. Dale CM, Sinuff T, Morrison LJ, Golan E, Scales DC. Understanding early decisions to withdraw life-sustaining therapy in cardiac arrest survivors. a qualitative investigation. Ann Am Thorac Soc. 2016; 13(7):1115–1122. PMID:
27073975.

8. Seki T, Tamura T, Suzuki M; SOS-KANTO 2012 Study Group. Outcome prediction of out-of-hospital cardiac arrest with presumed cardiac aetiology using an advanced machine learning technique. Resuscitation. 2019; 141:128–135. PMID:
31220514.

9. Kwon JM, Jeon KH, Kim HM, Kim MJ, Lim S, Kim KH, et al. Deep-learning-based out-of-hospital cardiac arrest prognostic system to predict clinical outcomes. Resuscitation. 2019; 139:84–91. PMID:
30978378.

10. Park JH, Shin SD, Song KJ, Hong KJ, Ro YS, Choi JW, et al. Prediction of good neurological recovery after out-of-hospital cardiac arrest: a machine learning analysis. Resuscitation. 2019; 142:127–135. PMID:
31362082.

11. Daou O, Winiszewski H, Besch G, Pili-Floury S, Belon F, Guillon B, et al. Initial pH and shockable rhythm are associated with favorable neurological outcome in cardiac arrest patients resuscitated with extracorporeal cardiopulmonary resuscitation. J Thorac Dis. 2020; 12(3):849–857. PMID:
32274152.

12. Kiehl EL, Amuthan R, Adams MP, Love TE, Enfield KB, Gimple LW, et al. Initial arterial pH as a predictor of neurologic outcome after out-of-hospital cardiac arrest: a propensity-adjusted analysis. Resuscitation. 2019; 139:76–83. PMID:
30946922.

13. Bender PR, Debehnke DJ, Swart GL, Hall KN. Serum potassium concentration as a predictor of resuscitation outcome in hypothermic cardiac arrest. Wilderness Environ Med. 1995; 6(3):273–282. PMID:
11990091.

14. Choi DS, Shin SD, Ro YS, Lee KW. Relationship between serum potassium level and survival outcome in out-of-hospital cardiac arrest using CAPTURES database of Korea: Does hypokalemia have good neurological outcomes in out-of-hospital cardiac arrest? Adv Clin Exp Med. 2020; 29(6):727–734. PMID:
32608580.

15. Tamura T, Suzuki M, Hayashida K, Sasaki J, Yonemoto N, Sakurai A, et al. Renal function and outcome of out-of-hospital cardiac arrest - multicenter prospective study (SOS-KANTO 2012 Study). Circ J. 2018; 83(1):139–146. PMID:
30333435.
16. D'Arrigo S, Cacciola S, Dennis M, Jung C, Kagawa E, Antonelli M, et al. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017; 121:62–70. PMID:
29020604.
17. Li DC, Hu SC, Lin LS, Yeh CW. Detecting representative data and generating synthetic samples to improve learning accuracy with imbalanced data sets. PLoS One. 2017; 12(8):e0181853. PMID:
28771522.

18. Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, et al. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015; 132(18):Suppl 2. S465–S482. PMID:
26472996.
19. Graf J, Mühlhoff C, Doig GS, Reinartz S, Bode K, Dujardin R, et al. Health care costs, long-term survival, and quality of life following intensive care unit admission after cardiac arrest. Crit Care. 2008; 12(4):R92. PMID:
18638367.

20. Efendijev I, Folger D, Raj R, Reinikainen M, Pekkarinen PT, Litonius E, et al. Outcomes and healthcare-associated costs one year after intensive care-treated cardiac arrest. Resuscitation. 2018; 131:128–134. PMID:
29958958.

21. Damluji AA, Al-Damluji MS, Pomenti S, Zhang TJ, Cohen MG, Mitrani RD, et al. Health care costs after cardiac arrest in the United States. Circ Arrhythm Electrophysiol. 2018; 11(4):e005689. PMID:
29654127.

22. Hong JY, Lee DH, Oh JH, Lee SH, Choi YH, Kim SH, et al. Grey-white matter ratio measured using early unenhanced brain computed tomography shows no correlation with neurological outcomes in patients undergoing targeted temperature management after cardiac arrest. Resuscitation. 2019; 140:161–169. PMID:
30953628.





 PDF
PDF Citation
Citation Print
Print



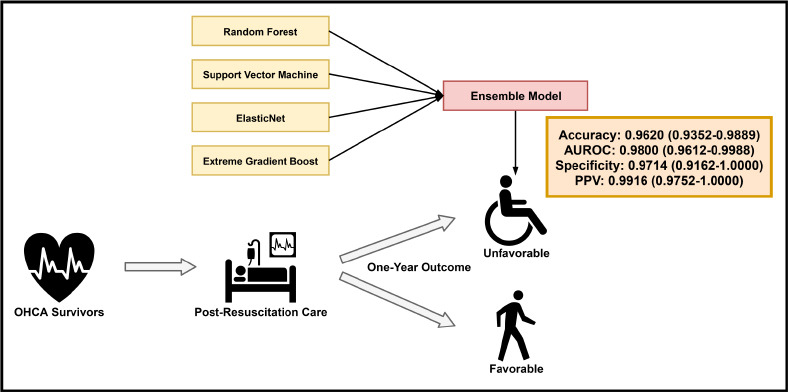
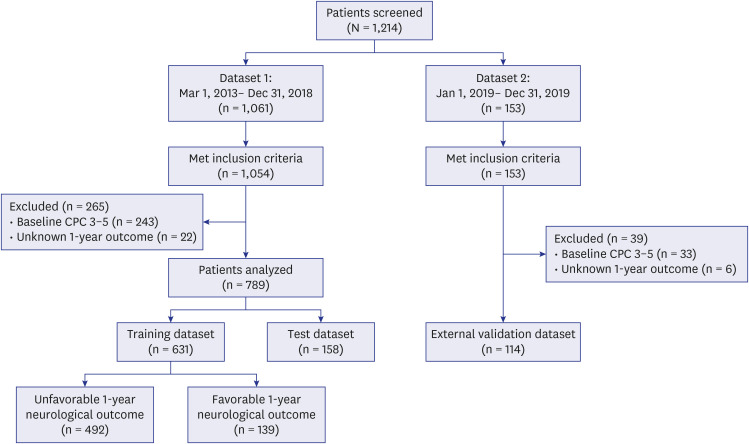
 XML Download
XML Download