Abstract
Although sexual difference medicine is a relatively new concept, it is currently an important research area because different treatments may be required according to gender. Gender-related differences in the epidemiology, progression, and treatment strategies of chronic liver disease has been known for a long time, but there are few meaningful studies in most epidemiologic and clinical studies, but gender differences in pharmacological treatment have been associated with dose, administration timing, and incidence of drug side effects. Until now, the liver expresses androgen and estrogen receptors, so there are gender differences due to gene expression patterns, immune responses, and metabolism depending on sex hormones.
Much work has been done in recent years to provide insight into the pathogenesis of alcoholic liver injury (ALI). Liver injury from alcohol has many components, including intrahepatic events (such as hepatocyte alcohol metabolism, generation of reactive oxygen species [ROS] that result in cellular oxidative stress, loss of protective enzymes and transporters) and extrahepatic stimuli, such as gut-derived endotoxin, induced by ethanol exposure. These factors can act separately or in concert to trigger common pathways involving an inflammatory cytokine cascade.1 These cytokines bring about interplay between different functional cell types in the liver, i.e. hepatocytes, Kupffer cells, stellate or Ito cells, mononuclear cells, and neutrophils. The outcome of such interplay between biochemical alterations and changes in stimuli is the induction of early metabolic dysfunction in the form of fatty liver, followed possibly by an inflammatory response (alcoholic hepatitis), and progression to a more progressive liver disease (fibrosis and cirrhosis).
The current model of ALI pathogenesis involves roles of different cytokines that induce various transcription factors, which in turn modulate expression of different genes. Contributing to this injury is mitochondrial dysfunction, with resultant reduced cellular ATP levels, increased oxidative stress brought about by mitochondrial injury and altered activities of enzymes such as CYP2E1 and NADPH oxidase, along with a decrease in protective antioxidant enzymes such as superoxide dismutase and glutathione peroxidase.2
Very little is known about possible direct effects of female sex hormones on gut permeability. A recent report shows that increased intestinal permeability is a factor in intrahepatic cholestasis of pregnancy; this finding is relevant in that pregnancy is characterized by high levels of estrogen and progesterone. A few studies have examined the effects of hormones on liver-enterotoxin interaction. When female rats are treated with a sublethal dose of endotoxin via tail vein, those rats also treated with a combination of synthetic estrogen and progesterone identical to that in oral contraceptives showed pronounced gut permeability and liver injury, with increased TNFα production by Kupffer cells.3 However, these studies used very high doses of hormones, and the hormones were only used as a combination, and not separately.4
In an alcohol model, treatment of female rats with very high doses of estriol, a very weak estrogen, resulted in increased endotoxin levels and increases in Kupffer cell TNFα and CD14 content.5 Because these effects were blocked with oral nonabsorbable antibiotics, the authors concluded that the estriol treatment resulted in increased gut permeability. In another study using burn and trauma/hemorrhagic models, it was shown that estrogen decreased markers of gut injury, whereas testosterone increased susceptibility. It should be noted that the mechanisms in these models may differ from that in ours, since in gut trauma models injury to the mucosal barrier is acute and severe, whereas in alcohol models the injury is chronic. Thus, hormonal changes in the acute gut trauma models may be entirely different than in the chronic alcohol models. In Caco-2 cells, estradiol treatment potentiated alcohol-induced apoptosis, leading to speculation that estrogen might increase gut permeability in vivo. With respect to the liver, an interesting study using cultured hepatic stellate cells showed that estradiol treatment reduced ROS generation through the NADPH oxidase system, and also attenuated TGFβ1 expression, cell activation, MAPK pathways, as well as fibrogenic responses. In contrast, progesterone treatment increased ROS generation, TGFβ1 expression, and stellate cell proliferation.6 Thus, a potential role of progesterone in ALI should be considered.
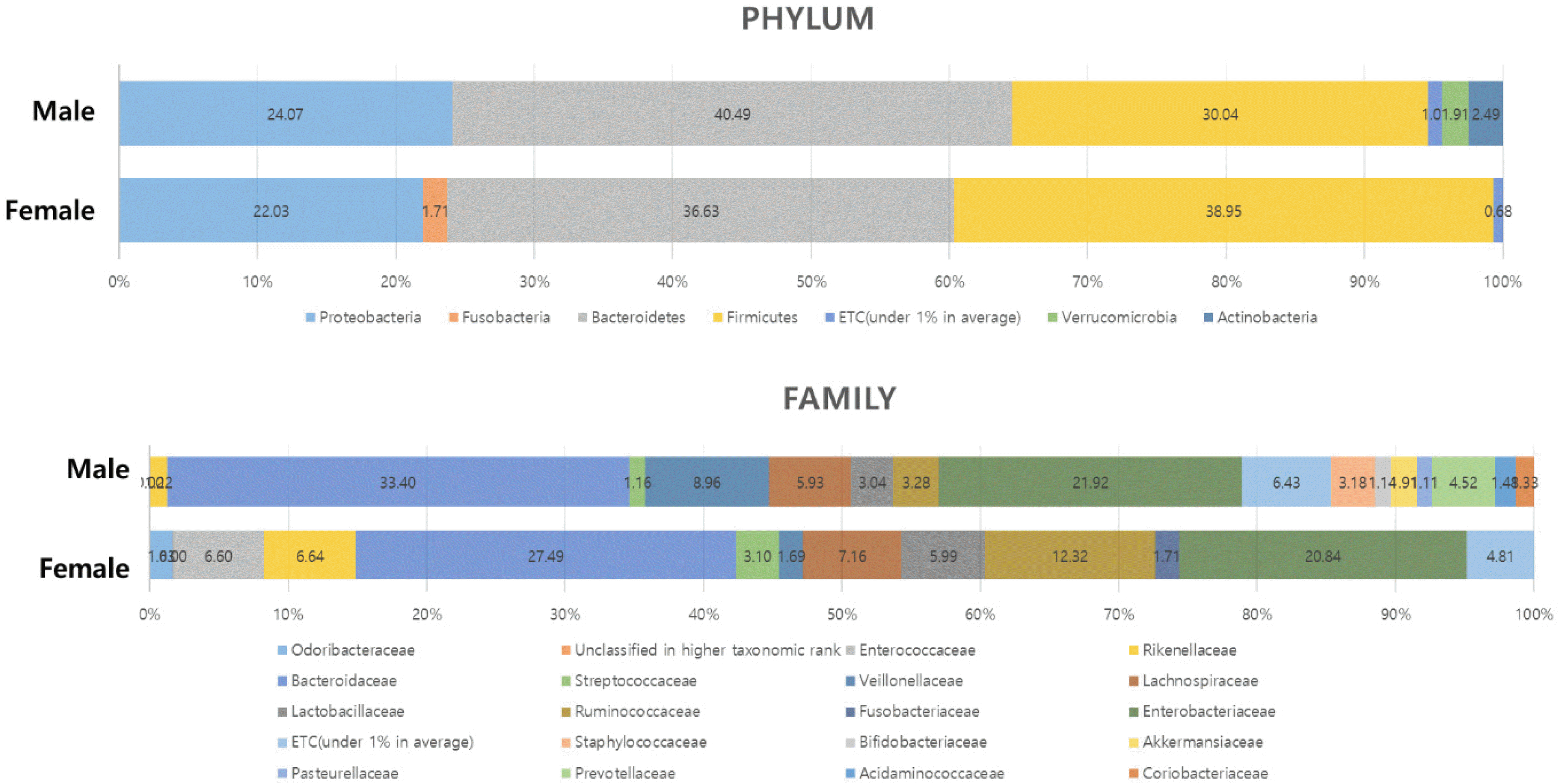
In microbiome analysis, genus Lactococcus and Leuconostoc, are more fluent in female. In alcoholic cirrhosis difference can be seen in the phyrum and family (Fig. 1).
The evidence strongly points to greater risk for women for developing severe ALI and cirrhosis than men. Although the exact roles of female hormones have not yet been proven or elucidated, women should exert caution by limiting their alcohol intake to one drink or less per day to avoid the complications of alcohol-induced liver injury.
REFERENCES
1. Lieber CS. 2004; Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 34:9–19. DOI: 10.1016/j.alcohol.2004.07.008. PMID: 15670660.

2. Tsukamoto H, Lu SC. 2001; Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 15:1335–1349. DOI: 10.1096/fj.00-0650rev. PMID: 11387231.

3. Konno A, Enomoto N, Takei Y, Hirose M, Ikejima K, Sato N. 2002; Oral contraceptives worsen endotoxin-induced liver injury in rats. Alcohol Clin Exp Res. 26 Suppl 8:70S–74S. DOI: 10.1097/00000374-200208001-00015.

4. Thurman RG. 1998; II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 275:G605–G611. DOI: 10.1152/ajpgi.1998.275.4.G605. PMID: 9756487.
5. Enomoto N, Yamashina S, Schemmer P, et al. 1999; Estriol sensitizes rat Kupffer cells via gut-derived endotoxin. Am J Physiol. 277:G671–G677. DOI: 10.1152/ajpgi.1999.277.3.G671. PMID: 10484393.
6. Itagaki T, Shimizu I, Cheng X, et al. 2005; Opposing effects of oestradiol and progesterone on intracellular pathways and activation processes in the oxidative stress induced activation of cultured rat hepatic stellate cells. Gut. 54:1782–1789. DOI: 10.1136/gut.2004.053728. PMID: 16284289. PMCID: PMC1774809.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download