Abstract
Colorectal perineuriomas are benign mucosal-based mesenchymal tumors composed of perineurial cells and show serrated or hyperplastic crypts in epithelium on histopathological evaluation. Most perineuriomas are usually presented as sessile polyps and often as subepithelial tumors. In this case, colonoscopy revealed a rectal subepithelial tumor (measuring approximately 7 mm) with yellowish-colored normal mucosa. A rectal neuroendocrine tumor was suspected, and cap-assisted endoscopic mucosal resection was performed. Histopathological examination of the resected specimen revealed bland spindle cells showing immunopositivity for CD34. The patient was finally diagnosed with rectal perineurioma.
Perineurioma, composed primarily of perineurial cells, is a rare benign tumor of the peripheral nerves. Colorectal perineurioma was first described by Eslami-Varzaneh et al.1 in 2004 as a benign fibroblastic polyp. Most colorectal perineuriomas usually occur as sessile polyps and often as subepithelial tumors.2,3 A colorectal perineurioma usually runs a benign course. However, in patients with subepithelial perineuriomas, the differential diagnosis should include gastrointestinal stromal tumors (GIST) and neuroendocrine tumors. To the best of our knowledge, no previous report in Korea has described rectal subepithelial perineurioma. We report a rare case of a rectal subepithelial perineurioma that mimicked a neuroendocrine tumor, which was accurately diagnosed after cap-assisted endoscopic mucosal resection.
A nonsmoking 58yearold man receiving medication for hypertension visited Inje University Haeundae Paik Hospital for colonoscopy screening. His vital signs were stable, with normal blood pressure and body temperature on initial outpatient examination. Physical examination and laboratory findings were unremarkable.
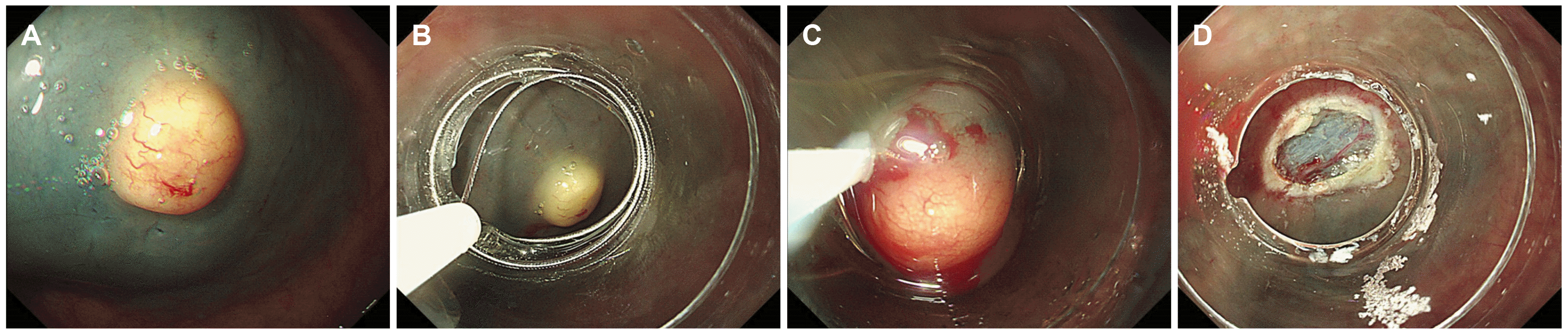
Colonoscopy revealed a rectal subepithelial lesion (approximately 7 mm in size) with yellowishcolored normal overlying mucosa (Fig. 1A). Narrowband imaging with magnification revealed dilated vessels on the mucosal surface, without any abnormal surface pattern (Fig. 1B). The application of pressure with forceps revealed that the lesion showed a positive rolling sign and negative cushion sign. Based on endoscopic findings, the lesion was highly suspected to be a rectal neuroendocrine tumor; however, diagnostic confirmation was not possible. We performed a cap-assisted endoscopic mucosal resection (EMR-C) for the suspected rectal neuroendocrine tumor. EUS was not performed because the tumor measured <1 cm and the patient refused this imaging examination. EMR-C was performed immediately after screening with colonoscopy, and en bloc resection was achieved (Fig. 2). No other endoscopic abnormalities were observed.
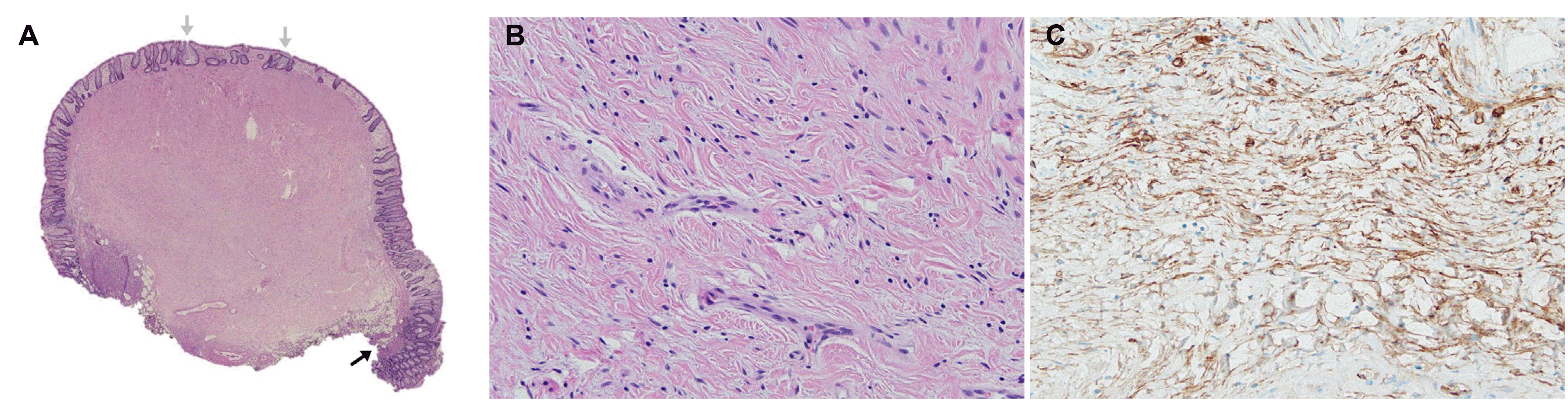
Histopathological evaluation revealed the following findings: low-power view showed a submucosal solid mass with low cellularity without necrotic or organoid cell nests (Fig. 3A). Hyperplastic polyp-like glandular hyperplasia was observed. High-magnification images showed bland spindle cells with oval nuclei and pale cytoplasm with indistinct cytological boundaries against a collagenous background (Fig. 3B). Cytological dimorphism or polymorphism was not identified, and no mitosis was observed. Lymphocytes were sparsely scattered throughout the lesion. Immunohistochemical evaluation revealed immunopositivity for CD34 (Fig. 3C) and immunonegativity for epithelial membrane antigen (EMA), c-kit, desmin, and S-100. Glucose transporter-1 (GLUT-1), claudin-1, and collagen type IV expression were not evaluated. The patient was finally diagnosed with rectal perineurioma.
A colorectal perineurioma, composed of perineurial cells, is a benign mucosal-based mesenchymal lesion showing serrated or hyperplastic crypts in epithelium on histopathological evaluation. Eslami-Varzaneh et al.1 first reported this lesion in 2004 as a benign fibroblastic polyp of the colon. It usually involves the rectosigmoid colon and commonly presents as a sessile polyp. Several reports have shown that the mean age at diagnosis is 60 years, and the lesion shows a slight female predominance.2 A colorectal perineurioma usually runs a benign course.
Endoscopically, colorectal perineuriomas manifest as well-circumscribed, sessile mucosal lesions, usually <1 cm in size. Most perineuriomas are usually presented as sessile polyps and often as subepithelial tumors. In histopathology, the tumor is characterized by intramucosal spindle cell proliferation with separation and distortion of colonic crypts, without cytological atypia or mitotic activity. Typical perineuriomas can be diagnosed based on endoscopic and histopathological findings.2 Groisman and Polak-Charcon4 suggested that accurate diagnosis requires detecting at least two immunohistochemical perineurial markers, including EMA, GLUT-1, claudin-1, and collagen type IV. GLUT-1 and claudin-1 show high sensitivity and specificity with strong immunoreactivity for perineural differentiation. EMA stains are often focal and weakly immunoreactive and may require a procedural variation to increase sensitivity.5 Based on molecular analysis studies performed in 2010, BRAF mutations are associated with the serrated crypt epithelium.6,7 In addition, Agaimy et al.6 performed molecular analysis and reported V600E BRAF mutation in 14 of 22 patients (63%) investigated, suggesting that the serrated epithelium is more likely a neoplastic development rather than a reactive phenomenon.
In our case, histopathological evaluation showed bland spindle cells with oval nuclei and pale cytoplasm with indistinct cytological boundaries against a collagenous background. Cytological dimorphism or polymorphism was not identified, and no mitosis was observed. The histopathological features were consistent with typical findings of a colorectal perineurioma. Although perineural markers for GLUT-1, claudin-1, and collagen type IV could not be analyzed due to the unavailability of the test kit at Inje University Haeundae Paik Hospital, immunohistochemical evaluations for c-kit, desmin, and S-100 were all negative. Therefore, we diagnosed the patient with perineurioma based on typical histopathological findings after other differential possibilities have been excluded.
Most colorectal perineuriomas are usually presented as sessile polyps and often as subepithelial tumors. In patients with subepithelial perineuriomas, the differential diagnosis should include GISTs and neuroendocrine tumors because of their malignant potential.8 Most subepithelial tumors are covered with normal mucosa, and diagnostic confirmation is challenging. Treatment methods and prognosis differ depending on the type of tumor; therefore, accurate differential diagnosis is essential. EUS is useful for diagnosing subepithelial tumors, although accurate identification may occasionally be challenging. EUS cannot be performed for all subepithelial tumors in clinical practice due to various factors, including equipment unavailability, economic costs, and patient preference. Therefore, endoscopic resection can be useful for diagnostic confirmation and resection of subepithelial tumors measuring <1 cm; modified EMR including EMR-C, EMR using a ligating device, and EMR after circumferential precutting are widely used in such cases. Based on endoscopic findings and size (<1 cm), we strongly suspected a rectal neuroendocrine tumor, and we performed EMR-C in our patient.
Colorectal perineuriomas are considered benign lesions; however, the epithelial components of serrated fibroblastic polyps may show BRAF mutations that may subsequently trigger fibroblast differentiation and proliferation.9 Because of the serrated variant’s neoplastic nature and the malignant potential of BRAF-positive serrated variants, postpolypectomy surveillance colonoscopy is important according to the guidelines for serrated colorectal polyps.10
Colorectal perineurioma is an uncommon, benign, mu-cosal-based mesenchymal lesion that usually presents as a sessile polyp and often as a subepithelial tumor. A subepithelial perineurioma can be misdiagnosed as a GIST or neuroendocrine tumor. Gastroenterologists and histopathologists should be familiar with the submucosal variety of perineuriomas. Immunohistochemical evaluation using perineurial markers such as EMA, GLUT-1, or claudin-1 is recommended in patients in whom an unusual mesenchymal tumor is suspected.
REFERENCES
1. Eslami-Varzaneh F, Washington K, Robert ME, Kashgarian M, Goldblum JR, Jain D. 2004; Benign fibroblastic polyps of the colon: a histologic, immunohistochemical, and ultrastructural study. Am J Surg Pathol. 28:374–378. DOI: 10.1097/00000478-200403000-00010. PMID: 15104300.

2. van Wyk AC, van Zyl H, Rigby J. 2018; Colonic perineurioma (benign fibroblastic polyp): case report and review of the literature. Diagn Pathol. 13:16. DOI: 10.1186/s13000-018-0694-z. PMID: 29463272. PMCID: PMC5819702.



3. Fujino Y, Muguruma N, Kitamura S, et al. 2014; Perineurioma in the sigmoid colon diagnosed and treated by endoscopic resection. Clin J Gastroenterol. 7:392–396. DOI: 10.1007/s12328-014-0519-x. PMID: 26184017.


4. Groisman GM, Polak-Charcon S. 2008; Fibroblastic polyp of the colon and colonic perineurioma: 2 names for a single entity? Am J Surg Pathol. 32:1088–1094. DOI: 10.1097/PAS.0b013e318160df3f. PMID: 18520438.


5. Hornick JL, Fletcher CD. 2005; Intestinal perineuriomas: clinicopathologic definition of a new anatomic subset in a series of 10 cases. Am J Surg Pathol. 29:859–865. DOI: 10.1097/01.pas.0000154130.87219.2c. PMID: 15958849.

6. Agaimy A, Stoehr R, Vieth M, Hartmann A. 2010; Benign serrated colorectal fibroblastic polyps/intramucosal perineuriomas are true mixed epithelial-stromal polyps (hybrid hyperplastic polyp/mucosal perineurioma) with frequent BRAF mutations. Am J Surg Pathol. 34:1663–1671. DOI: 10.1097/PAS.0b013e3181f4a458. PMID: 20962618.

7. Groisman GM, Hershkovitz D, Vieth M, Sabo E. 2013; Colonic perineuriomas with and without crypt serration: a comparative study. Am J Surg Pathol. 37:745–751. DOI: 10.1097/PAS.0b013e318277a1a9. PMID: 23588369.

8. McCarthy AJ, Karamchandani DM, Chetty R. 2018; Neural and neurogenic tumours of the gastroenteropancreaticobiliary tract. J Clin Pathol. 71:565–578. DOI: 10.1136/jclinpath-2017-204895. PMID: 29419412.


9. Kolli S, Gujjula S, Ona MA. 2019; Colonic perineurioma's malignant proximity to serrated colonic polyps. Cureus. 11:e4815. DOI: 10.7759/cureus.4815. PMID: 31404377. PMCID: PMC6682396.

10. Jama GM, Evans M, Fazal MW, Singh-Ranger D. 2018; Perineurioma of the sigmoid colon. BMJ Case Rep. 2018:bcr2018227170. DOI: 10.1136/bcr-2018-227170. PMID: 30262546. PMCID: PMC6169695.

Fig. 1
Endoscopic imaging findings. (A) Image showing a lobulated rectal lesion with yellowish-colored normal mucosa. (B) Narrow-band image with magnification showing dilated vessels on the mucosal surface without any specific surface pattern.
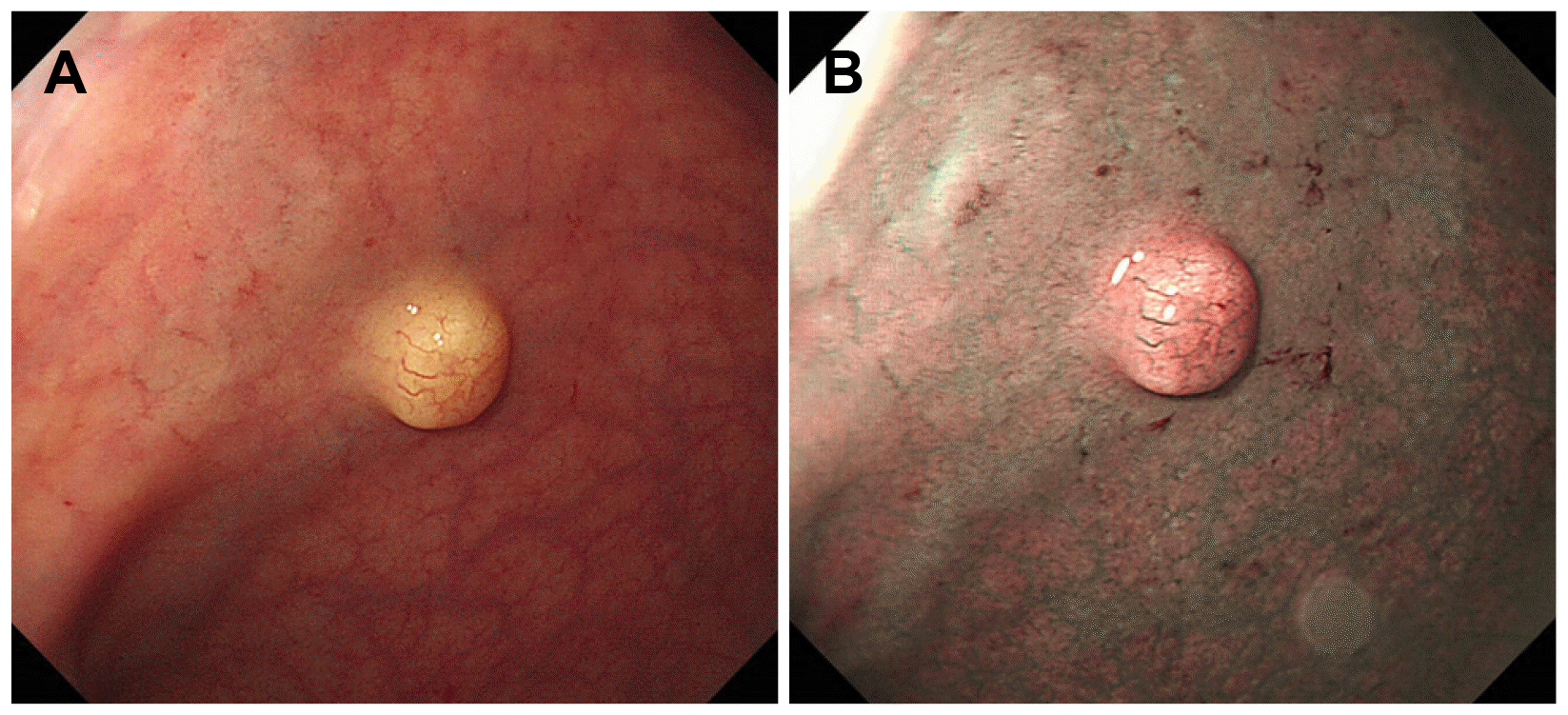
Fig. 3
Histopathological findings. (A) Low-magnification view (hematoxylin-eosin stain) showing a solid mass with low cytoplasm centered on the submucosa. The black arrow indicates the fascia mucosa that separates the mucous membrane from the submucosa. Gray arrows indicate hyperplastic glands (hematoxylin-eosin stain, ×1). (B) High-magnification view showing spindle cell proliferation with cells containing oval nuclei and pale cytoplasm against a collagenous background. No cytological dimorphism, polymorphism, or mitosis is observed (hematoxylin-eosin stain, ×200). (C) Immunohistochemical evaluation showing spindle cells with immunopositivity for the CD34 antibody (CD34 stain ×200).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download