Dear Editor,
The genus Bordetella comprises 12 species, some of which cause human diseases. Bordetella hinzii widely exists in poultry and rodents and has occasionally been reported in humans wherein it has been confirmed as a causative agent of pulmonary and digestive infection, and bacteremia, almost invariably in patients with immunodeficiency [1,2]. Only 11 cases of human B. hinzii infection have been reported to date, and none has been found in Asia (Table 1). However, the clinical and epidemiologic characteristics of B. hinzii infection remain to be determined. Here, we describe the first Asian case of B. hinzii pneumonia to highlight the pathogenicity of this bacterium. As this case was identified during a routine surveillance organized by the Centers for Disease Control and Prevention, the need for ethical approval for the present study was exempted by the Institutional Review Board of Chengdu Fifth People’s Hospital, China; verbal consent was obtained from the patient for case presentation.
A 67-year-old woman was admitted to the Neurological Intensive Care Unit in Chengdu Fifth People’s Hospital on January 8, 2013 because of fatigue and loss of the abilities to stand, walk, and speak clearly. Computed tomography (CT) showed spontaneous intracerebral hemorrhage involving the ventricles of the brain. The patient had been suffering from type 2 diabetes in the past year but had no other remarkable medical history. After admission, she received symptomatic treatment, supplementary fluids, and antihypertensive treatment. She developed fever on the third day. Her vital signs included a temperature of 38°C; blood pressure, 148/70 mm Hg; pulse, 84 beats/min; and respiratory rate, 27 breaths/min. Routine blood examination showed a leukocyte count of 9.27×109/L; neutrophil percentage, 88.1%, lymphocyte percentage, 6.5%; platelet count, 91×109/L; and high sensitive C-reactive protein level, 28.9 mg/L. Chest CT examination showed scattered turbidity in both lungs. Therefore, a pulmonary infection was suspected and intravenous cefmetazole (1.5 g daily) was empirically selected and initiated. The patient’s condition improved, and she was discharged on January 15, 2013.
The blood culture result was negative. Direct gram staining and microscopic examination of the sputum revealed gram-negative rods in the neutrophils. After 18 hours incubation at 35°C, colorless colonies were detected on blood, chocolate, and MacConkey agar plates, which were all gram-negative rods. One isolate from each plate was selected for identification. Initial identification performed using the API 20NE strip (bioMérieux, Lyons, France) classified the isolate as Bordetella avium with a 96.6% confidence, which could not be used to distinguish species in the genus Bordetella. The selected isolate from the blood agar plate, designated strain A2799, was subject to further identification using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) (Bruker, Leipzig, Germany) and 16S rRNA gene sequencing. Both methods classified this strain as B. hinzii. Antibiotic susceptibility testing was performed using the Kirby-Bauer method. The strain was found to be susceptible to the most commonly used antibiotics, and our patient recovered well through therapy with cefmetazole, which is similar to cefoxitin and ceftazidime, to which the strain was found to be susceptible.
Furthermore, the whole-genome sequence of A2799 (GenBank accession no. SRP081450) was obtained. Phylogenetic trees were constructed using a distance matrix based on the presence or absence of genes (pan-genome tree) and single nucleotide polymorphisms in the core genome (core-genome tree) for A2799 and 27 other Bordetella strains (Fig. 1). In both trees, A2799 clustered with two B. hinzii strains and with B. pseudohinzii, further suggesting that A2799 is B. hinzii. The three “classical” Bordetella species, B. pertussis, B. parapertussis, and B. bronchiseptica, were closely associated in both the pan-genome and core-genome phylogenetic trees, indicating that these three species were derived from a recent common ancestor (Fig. 1).
Our patient had been suffering from cerebral hemorrhage but had no history of physical injury or trauma on admission. In patients with B. hinzii infections, the medical history often reveals a recent exposure to poultry. However, there was a history of avian exposure for this patient. Thus, the exact nature of the exposure in our patient remains unknown.
The identification of B. hinzii was inconclusive or inaccurate with a biochemical identification system, consistent with a previous case [1]. 16S rRNA gene sequencing and routine MALDI-TOF-MS are reliable for identifying B. hinzii [1]. Furthermore, whole-genome sequencing is increasingly being applied to identify clinical microorganisms. Therefore, it is likely that some species that could not previously be identified by traditional biochemical methods will increasingly be discovered. B. hinzii infection is rare but potentially fatal. However, antibiotic therapy often results in a favorable outcome.
Notes
AUTHOR CONTRIBUTIONS
Zhou H designed the study. Chen D, wang H, Lu X performed the data collection and sampling and carried out the experiments. Cui Y, Ma X, Lou J, Zhou H carried out the sequencing and data analysis. Chen D, Lou J and Zhou H wrote the manuscript. All authors read and approved the final version of the manuscript.
REFERENCES
1. Fabre A, Dupin C, Bénézit F, Goret J, Piau C, Jouneau S, et al. 2015; Opportunistic pulmonary Bordetella hinzii infection after avian exposure. Emerg Infect Dis. 21:2122–6. DOI: 10.3201/eid2112.150400. PMID: 26584467. PMCID: PMC4672423.


2. Vandamme P, Hommez J, Vancanneyt M, Monsieurs M, Hoste B, Cookson B, et al. 1995; Bordetella hinzii sp. nov., isolated from poultry and humans. Int J Syst Bacteriol. 45:37–45. DOI: 10.1099/00207713-45-1-37. PMID: 7857806.

3. Funke G, Hess T, von Graevenitz A, Vandamme P. 1996; Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J Clin Microbiol. 34:966–9. DOI: 10.1128/JCM.34.4.966-969.1996. PMID: 8815118. PMCID: PMC228927.


4. Cookson BT, Vandamme P, Carlson LC, Larson AM, Sheffield JV, Kersters K, et al. 1994; Bacteremia caused by a novel Bordetella species, "B. hinzii". J Clin Microbiol. 32:2569–71. DOI: 10.1128/JCM.32.10.2569-2571.1994. PMID: 7814500. PMCID: PMC264104.


5. Kattar MM, Chavez JF, Limaye AP, Rassoulian-Barrett SL, Yarfitz SL, Carlson LC, et al. 2000; Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J Clin Microbiol. 38:789–94. DOI: 10.1128/JCM.38.2.789-794.2000. PMID: 10655386. PMCID: PMC86205.
6. Gadea I, Cuenca-Estrella M, Benito N, Blanco A, Fernandez-Guerrero ML, Valero-Guillen PL, et al. 2000; Bordetella hinzii, a "new" opportunistic pathogen to think about. J Infect. 40:298–9. DOI: 10.1053/jinf.2000.0646. PMID: 10908033.

7. Arvand M, Feldhues R, Mieth M, Kraus T, Vandamme P. 2004; Chronic cholangitis caused by Bordetella hinzii in a liver transplant recipient. J Clin Microbiol. 42:2335–7. DOI: 10.1128/JCM.42.5.2335-2337.2004. PMID: 15131227. PMCID: PMC404647.


8. Fry NK, Duncan J, Edwards MT, Tilley RE, Chitnavis D, Harman R, et al. 2007; A UK clinical isolate of Bordetella hinzii from a patient with myelodysplastic syndrome. J Med Microbiol. 56:1700–3. DOI: 10.1099/jmm.0.47482-0. PMID: 18033844.

9. Hristov AC, Auwaerter PG, Romagnoli M, Carroll KC. 2008; Bordetella hinzii septicemia in association with Epstein-Barr virus viremia and an Epstein-Barr virus-associated diffuse large B-cell lymphoma. Diagn Microbiol Infect Dis. 61:484–6. DOI: 10.1016/j.diagmicrobio.2008.03.013. PMID: 18482816.

10. Palacián Ruiz MP, Vasquez Martinez MA, Lopez Calleja AI. 2013; Respiratory infection caused by Bordetella hinzii. Arch Bronconeumol. 49:409–10. DOI: 10.1016/j.arbres.2013.02.001. PMID: 23755859.

Fig. 1
Phylogenetic trees based on whole-genome sequencing and analysis. (A) Phylogenetic tree based on the gene content (pan-genome tree). (B) Phylogenetic tree based on 810 core-genome single nucleotide polymorphisms (core-genome tree).
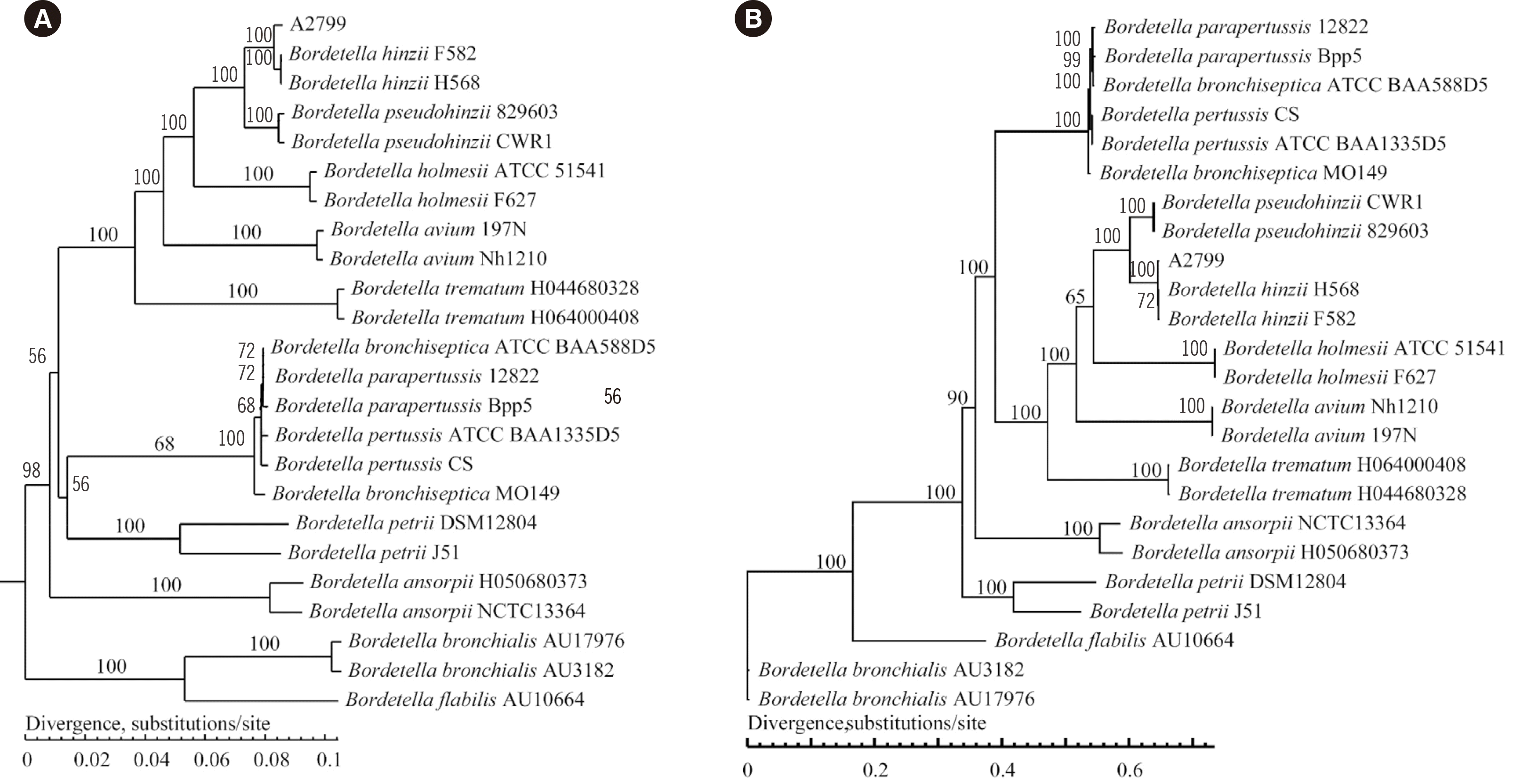
Table 1
Previous cases of human infection caused by Bordetella hinzii
| Year | Country | Clinical diagnosis | Gender | Age | Epidemiology | Immunosuppression status | Method of strain identification | Antibiotics treated | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1957 | France | NR | NR | NR | NR | NR | Identification of new species through PAGE of whole-cell proteins, Fatty acid methyl ester analysis, DNA-DNA and DNA-rRNA hybridization | NR | NR | [2] |
| 1992 | Switzerland | Pulmonary symptoms | Male | 51 | NR | None | API 20 NFT (NE) system, alkali production from malonate and PAGE of whole-cell proteins | Amoxicillin-clavulanic acid, ciprofloxacin | Recovered | [3] |
| 1994 | United States | Bacteremia | Male | 42 | NR | Immunosuppressed (AIDS) | API NFT, whole-cell fatty acid analysis and DNA-DNA hybridization | Vancomycin; ceftriaxone; ceftriaxone, rifampin | Recovered | [4] |
| 1999 | United States | Cholestasis and bacteremia | Male | 69 | Had attended a cookout at a farm 2 weeks before admission | None | Traditional biochemical testing and 16S rRNA gene sequence analysis | Ampicillin-sulbactam, cefotetan; ampicillin, gentamicin, metronidazole; ticarcillin-sulbactam, ciprofloxacin | Died | [5] |
| 2000 | Spain | Respiratory tract infection | NR | NR | No avian exposure | Immunosuppressed (AIDS) | Traditional biochemical testing and 16S rRNA gene sequence analysis | NR | NR | [6] |
| 2001 | Germany | Chronic cholangitis | Male | 29 | NR | Immunosuppressed (liver transplant recipient) | Traditional biochemical testing and 16S rRNA gene sequence analysis | Piperacillin-tazobactam, gentamicin; amphotericin B, flucytosine, vancomycin, and meropenem | Died | [7] |
| 2007 | United Kingdom | Flu-like symptoms | Male | 79 | NR | Immunosuppressed (myelodysplastic syndrome) | Genotypic methods and gene sequence analysis (ompA, 16S rRNA gene) | Amoxicillin, clavulanic acid; vancomycin, ceftazidime | Recovered | [8] |
| 2008 | United States | Fevers, full-body rash, fatigue; respiratory distress | Female | 36 | NR | Immunosuppressed (Epstein–Barr virus viremia and lymphoma) | Cellular fatty acid analysis and 16S rRNA gene sequence analysis | Amoxicillin-clavulanic acid, oxacillin, vancomycin, trimethoprim–sulfamethoxazole, doxycycline, linezolid, meropenem, itraconazole. | Died | [9] |
| 2013 | Spain | Respiratory symptoms | Female | 85 | NR | NR | MALDI-TOF–MS; 16S rRNA gene sequence analysis | Amoxicillin-clavulanate | Unclear | [10] |
| 2013 | France | Pulmonary infection | Male | 43 | Avian exposure | Immunosuppressed (leukemia, diabetes, vascular hypertension, and non-symptomatic chronic bronchiectasis before the allograft) | MALDI-TOF-MS; 16S rRNA gene sequence analysis | Ciprofloxacin; trimethoprim/sulfamethoxazole; piperacillin/tazobactam, ciprofloxacin; vancomycin | Recovered | [1] |
| 2014 | France | Chronic obstructive pulmonary disease | Male | 74 | No recent exposure to pets and poultry | Immunosuppressed (vascular hypertension, dyslipidemia, prostate cancer, ischemic heart disease) | MALDI-TOF-MS; 16S rRNA gene sequence analysis | Piperacillin/tazobactam, vancomycin | Recovered | [1] |
| 2013 | China | Pneumonia | Female | 67 | No avian exposure | Cerebral hemorrhage | MALDI-TOF-MS; 16S rRNA gene sequence analysis | Cefmetazole | Recovered | This study |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download