Abstract
Notes
REFERENCES
Fig. 1
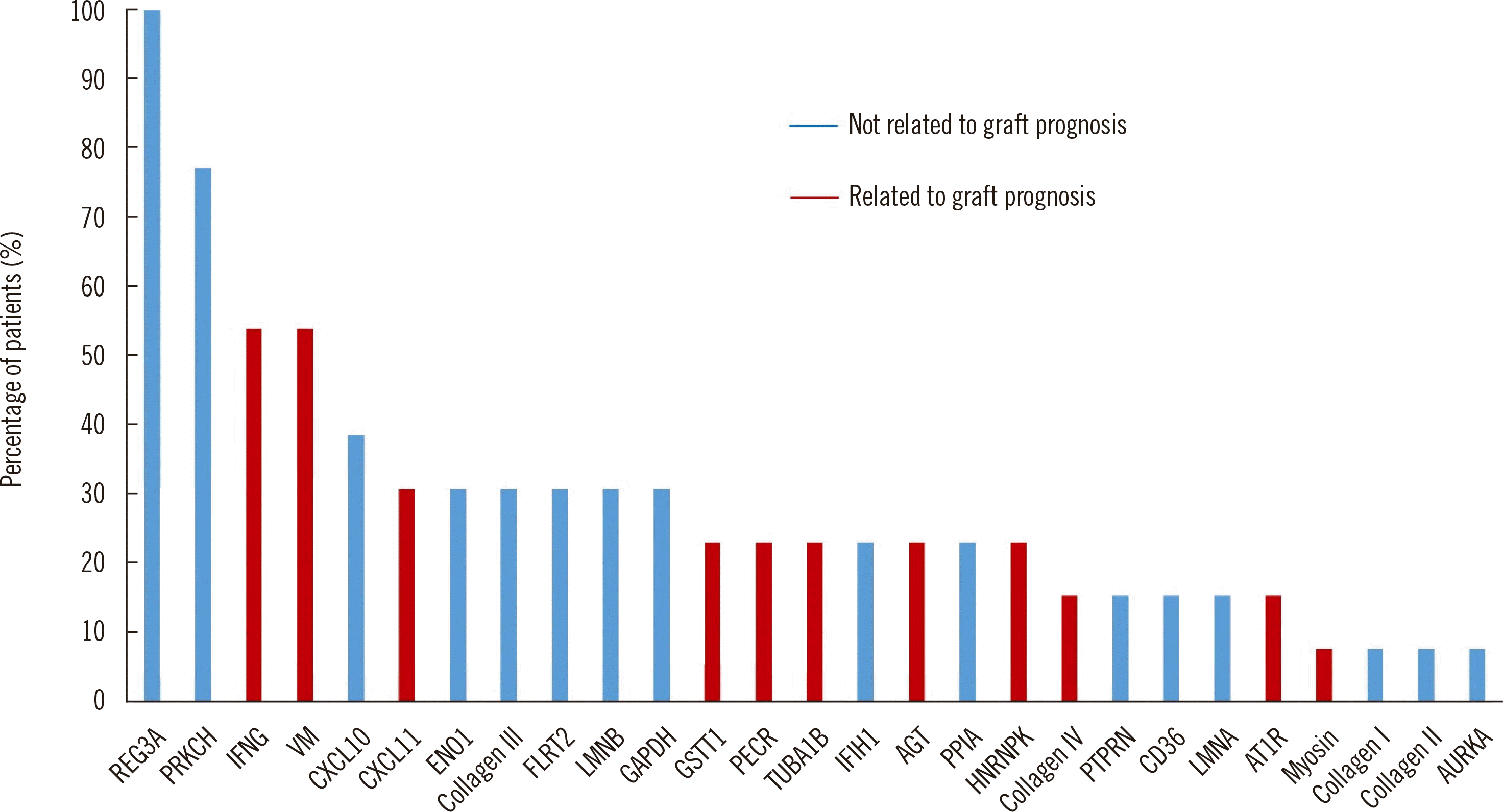
Table 1
| T-CDC* | T-AHG* | B-CDC* | T-FCXM† | B-FCXM† | N (%) patients (N=251) | DSAs present (N=88) | DSAs absent (N=163) | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| N (%) patients | Class I DSAs‡ | Class II DSAs‡ | N (%) patients | ||||||
| N | N | N | N | P 2.3 (1.3−9.0) | 17 (6.8) | 10 (11.4) | 3,458 (1,377–7,445) [5] | 11,628 (2,875–37,537) [7] | 7§ (4.3) |
| N | N | N | P 5.0 (NA) | N | 1 (0.4) | 0 (0.0) | – | – | 1∥ (0.6) |
| N | N | N | P 3.6 (1.3−13.6) | P 3.6 (1.7−33.6) | 10 (4.0) | 8 (9.1) | 2105 (1,071–9,740) [8] | 5,541 (2,301–8,781) [2] | 2 (1.2) |
| N | N | P 1:4 (NA) | N | N | 1 (0.4) | 0 (0.0) | - | - | 1∥ (0.6) |
| N | N | P 1:32 (1:1−1:32) | N | P 3.0 (1.47−70.47) | 194 (77.3) | 45 (51.1) | 2,541 (1,140–17,458) [21] | 6,827 (1,078–28,471) [31] | 149¶ (91.4) |
| N | N | P 1:4 (1:2−1:32) | P 3.7 (1.4−5.9) | P 16.7 (1.7−36.7) | 5 (2.0) | 3 (3.4) | 4,768 (1253–8283) [2] | 16,633.50 (5,756–27,511) [2] | 2 (1.2) |
| N | P | N | P 1.25 (NA) | P 1.87 (NA) | 1 (0.4) | 1 (1.1) | 14,084 | - | 0 (0.0) |
| N | P 1:18 (1:1−1:32) | P 1:18 (1:2−1:32) | P 2.0 (1.3−29.4) | P 2.9 (2.5−29.5) | 6 (2.4) | 6 (6.8) | 15,154.50 (4,022–32,991) [6] | 6,688.50 (4,239–9,138) [2] | 0 (0.0) |
| P 1:4 (1:1−1:32) | P 1:4 (1:1−1:32) | P 1:4 (1:2−1:32) | P 3.0 (1.3−94.95) | P 5.5 (1.6−119.2) | 16 (6.4) | 15 (17.0) | 16,178 (4,000–55,207) | 9,040 (1,037–29,293) | 1 (0.6) |
*Median (range) titers are shown for positive results.†Median (range) values of MFI from positive patients. There was no titer and 95% confidence interval (CI) value for only one positive patient. ‡Median (range) MFI values of DSAs (number of patients). §B-CDC(−)/B-FCXM(+) patients. One of seven patients received rituximab treatment and were considered to have a rituximab-induced positive result. ¶B-CDC(+)/B-FCXM(+) patients. All 149 patients received rituximab treatment and were considered to have a rituximab-induced positive result. ∥Repeat tests showed the same results in the two patients with T-FCXM (+) only and B-CDC (+) only. Technical error could not be ruled out.
Table 2
| Patient. | Sex/age | CDC | T/B-FCXM | HLA Ab (l/ll) | Non-HLA Abs | Sensitization Hx | TP | Duration of follow up (month) | Clinical course |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/43 | N/N/N* | N/P† (1.4) | P/P | VM (2,388)‡, REG3A (151) | Pregnancy | LDKT | 21 | Stable |
| 2¶ | F/48 | N/N/N | P(1.3)/P(1.7) | P/N | PRKCH (2,825), CXCL11 (400), IFNG (584), REG3A (310), Collagen II (453) | Pregnancy | LDKT | 15 | Rejection (5 months post-KT)§ |
| 3 | F/54 | N/N/N | N/P(2.8) | N/N | AT1R, VM (1267), GSTT1 (12,718), PRKCH (2,319), IFNG (955), REG3A (126) | Transfusion | LDKT | 9 | Stable |
| 4 | M/58 | N/N/P(1:4) | P(5.9)/P(4.6) | N/P | ENO1 (4,710), PRKCH (1279), CXCL11 (393), CXCL10 (375), IFNG (816), REG3A (571), GAPDH (1363), Collagen III (253) | Transfusion | LDKT | 7 | Stable |
| 5 | M/45 | N/N/N | P(5.1)/P(4.6) | P/N | REG3A (101) | - | LDKT | 6 | Stable |
| 6 | M/50 | N/N/N | N/P(3.0) | P/N | PRKCH (2,914), REG3A (125) | - | LDKT | 5 | Not done |
| 7** | M/61 | P(1:1)/P(1:2)/P(1:2) | P(2.1)/P(5.2) | N/N | VM (1,070), GSTT1 (6,594), PECR (5,035), PRKCH (1491), LMNB (2,851), REG3A (865), GAPDH (836) | - | LDKT | 4 | Rejection episode (1 month post-KT)∥ |
| 8 | M/66 | N/N/N | P(5.0)/N | N/N | ENO1 (6,704), FLRT2 (4,665), VM (9,890), TUBA1B (4,802), CD36 (3,074), IFIH1 (6,991), AGT (10,139), AURKA (4,995), PPIA (7,882), GSTT1 (8,373), LMNA (8,198), PECR (5,503), PRKCH (5,931), LMNB (6,927), CXCL11 (406), CXCL10 (328), HNRNPK (1274), IFNG (724), REG3A (417), Collagen III (152), Collagen IV (74) | Transfusion | LDLT | 8 | Stable |
| 9 | M/67 | N/N/N | N/P(3.4) | N/N | ENO1 (13,482), PECR (6,876), PRKCH (1204), REG3A (265), Collagen III (167), Collagen IV (48) | Transfusion | LDLT | 5 | Stable |
| 10 | M/56 | N/N/N | N/P(3.2) | N/P | ENO1 (5,236), IFIH1 (12,009), PTPRN (3,377), PRKCH (1511), CXCL11 (338), CXCL10 (360), HNRNPK (1,136), IFNG (897), REG3A (311) | Transfusion | LDLT | 12 | Stable |
| 11 | M/64 | N/N/P(1:4) | N/N | N/P | FLRT2 (5,684), VM (9,281), TUBA1B (4,086), CD36 (4,163), IFIH1 (4,386), AGT (6,010), PTPRN (4,800), PPIA (5,209), LMNA (8,295), PRKCH (1602), LMNB (6,397), CXCL10 (506), HNRNPK (1670), IFNG (618), REG3A (253), GAPDH (2,454) | - | LDLT | 10 | Stable |
| 12 | M/32 | N/N/P(1:2) | P(3.7)/P(1.7) | P/P | AT1R, ENO1 (4,635), FLRT2 (2,209), VM (7,134), TUBA1B (2,695), Myosin (9,554), AGT (5,915), PPIA (4,187), PRKCH (7,632), LMNB (3,143), HNRNPK (1824), IFNG (797), REG3A (553), GAPDH (1445), Collagen I (592), Collagen III (404) | Transfusion | LDLT | 10 | Stable |
| 13 | F/17 | N/N/N | N/P(2.2) | N/N | FLRT2 (749), VM (1849), CXCL10 (378), REG3A (207) | - | LDKT | 7 | NA |
*T-CDC, T-AHG, B-CDC in order (highest titer value in positive CDC is given in parenthesis). †T-FCXM, B-FCXM in order (MFI given in parenthesis). ‡MFI value of raw trimmed mean minus negative control bead [reference background values of lower 95% non-transplant population; ENO1 (4,218), FLRT2 (688), VM (820), TUBA1B (1987), CD36 (1591), IFIH1 (3,870), MYOSIN (9,341), AGT (1641), PTPRN (3,042), AURKA (4,892), CHAF1B (11,722), PPIA(3,292), EIF2A (6,901), GSTT1 (6,136), LMNA (6,633), PRKCZ (9,104), PECR (4,120), PRKCH (1,048), LMNB (2065), CXCL11 (309), CXCL10 (285), CXCL9 (575), AGRIN (504), ARHGDIB (3,918), GDNF (1,004), HNRNPK (845), IFNG (498), NCL (3,669), REG3A (86), GAPDH (508), TNFA (5,331), PLA2R (195), LG3 (3,801), Collagen I (375), Collagen II (155), Collagen III (128), Collagen IV (71), Collagen V (187), Fibronectin (141)]. §Non-HLA antibodies at the time of rejection; PRKCH (1298), REG3A (162), Collagen II (161). ∥Non-HLA antibodies at the time of rejection; VM (1595), AGT (1739), PPIA (3,434), PECR (4,684), PRKCH (1955), IFNG (662), REG3A (510). ¶Patient 2 developed biopsy-confirmed T cell-mediated rejection at five months post-KT. **Patient 7 presented with an elevated creatinine level one month post-KT; subsequent biopsy revealed C4d deposition with acute tubular necrosis.
Abbreviations: XM, crossmatch; M, Male; F, Female; DSAs, donor-specific antibodies; CDC, complement-dependent cytotoxicity; FCXM, flow cytometry crossmatch; HLA, human leukocyte antigen; Ab, antibody; Hx, history; TP, transplantation; N, negative; P, positive; MFI, mean fluorescent intensity; VM, vimentin; REG3A, regenerating islet-derived protein 3-alpha; LDKT, living donor kidney transplantation; PRKCH, protein kinase C eta type; CXCL11, C-X-C motif chemokine 11; IFNG, interferon gamma; AT1R, angiotensin II type 1 receptor; GSTT1, glutathione S-transferase theta-1; ENO1, alpha-enolase; CXCL10, C-X-C motif chemokine 10; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KT, kidney transplantation; PECR, peroxisomal trans-2-enoyl-CoA reductase; PTPRN, receptor-type tyrosine-protein phosphatase-like N; LMNB, lamin-B1; FLRT2, leucine-rich repeat transmembrane protein; TUBA1B, tubulin alpha-1B chain; IFIH1, interferon-induced helicase C domain-containing protein 1; AGT, angiotensinogen; AURKA, aurora kinase A-interacting protein; PPIA, peptidyl-prolyl cis-trans isomerase A; LMNA, prelamin-A/C; HNRNPK, heterogeneous nuclear ribonucleoprotein K; LDLT, living donor liver transplantation; NA, not applicable; AHG, anti-human globulin antibody; CHAF1B, chromatin assembly factor 1 subunit B; EIF2A, eukaryotic translation initiation factor 2A; PRKCZ, protein kinase C zeta; ARHGDIB, Rho GDP dissociation inhibitor beta; GDNF, glial cell line-derived neurotrophic factor; NCL, nucleolin; TNFA, tumor necrosis factor alpha; PLA2R, phospholipase A2 receptor.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download