Abstract
Background
Methods
Results.
Conclusions
Notes
AUTHOR CONTRIBUTIONS
Kim SY contributed to the data acquisition, prepared the first draft of the manuscript, and edited the manuscript draft. Shin CH and Shin CH contributed to data acquisition. Yang SW and Cho TJ made substantial contributions to genetic data acquisition and interpretation. Ko JM edited the manuscript drafts until the final draft was produced and mentored Kim SY by correspondence.
REFERENCES
Fig. 1
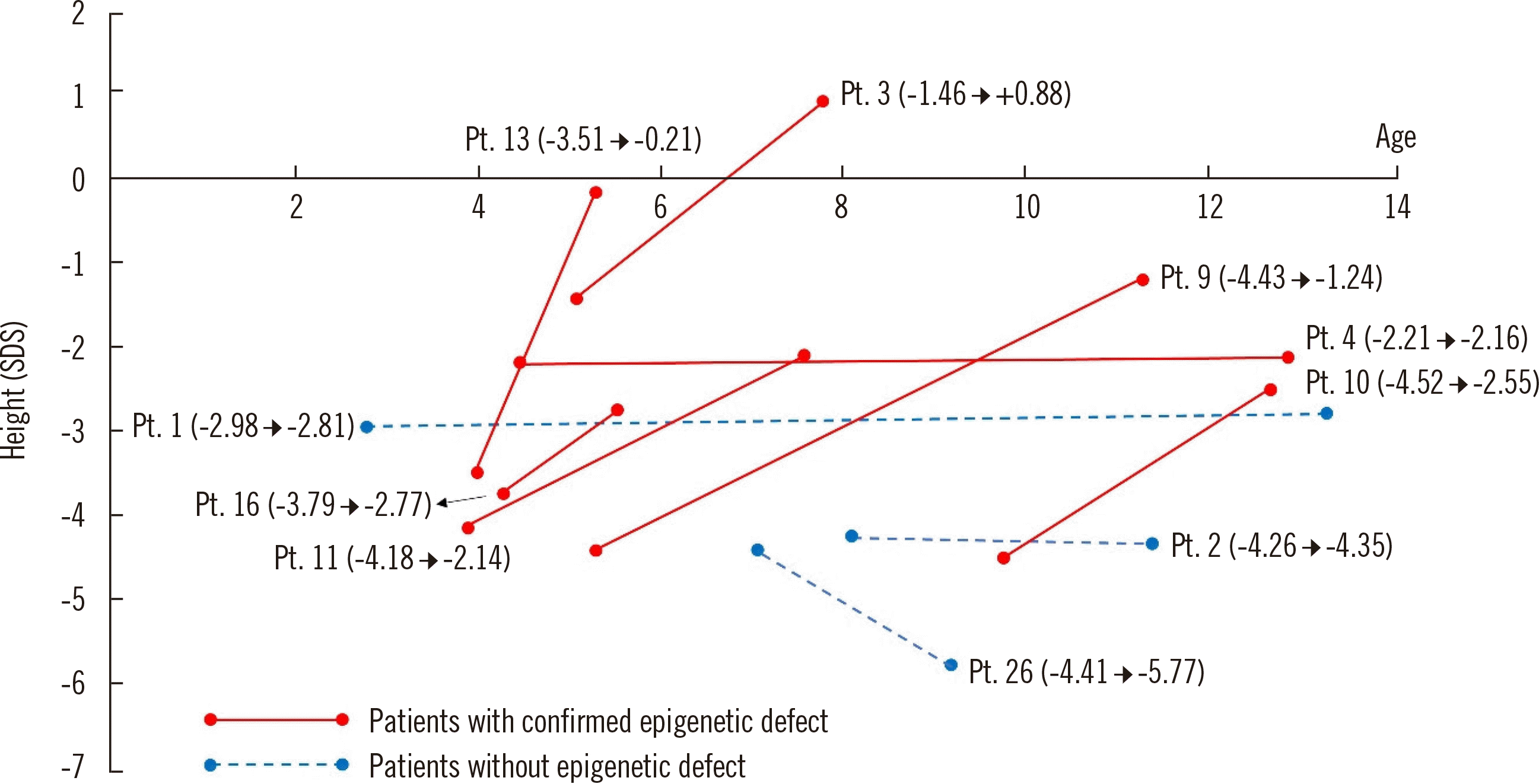
Table 1
Table 2
| No. case | Gender | Gestation (weeks, days) | Birth weight (SDS) | MPH (SDS) | Last follow-up visit | Clinical manifestations | Molecular analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Age (month) | GH Tx (months of duration) | Height (SDS) | Weight (SDS) | Key characteristics of SRS | Other characteristics | MS-MLPA for 11p15 | Bisulfite pyrosequencing* | |||||||
|
|
||||||||||||||
| IC1 (%mC) | IC2 (%mC) | MEST (%mC) | ||||||||||||
| 1 | Female | 39, 0 | −2.20 | −0.84 | 171 | 138 | −2.81 | −4.55 | PE, C, F | Normal | N | N | N | |
| 2 | Male | 39, 0 | −2.26 | 0.63 | 137 | 43 | −4.35 | −2.10 | TF, M, C | Normal | N | N | N | |
| 3 | Female | 40, 0 | −2.61 | 1.35 | 135 | 30 | 0.47 | 1.66 | M, C, LLD | IC1-LOM | L (33.6) | N | N | |
| 4 | Male | 39, 6 | −4.11 | −0.26 | 155 | 100 | −2.16 | −3.14 | TF, FB, M, C, LLD, F | SD, MD | IC1-LOM | L (14.0) | N | N |
| 5 | Female | 39, 0 | −2.48 | 1.46 | 63 | ND | −2.66 | −3.35 | M, C, LLD | Normal | N | N | N | |
| 6 | Male | 39, 0 | −2.09 | −0.08 | 9 | ND | −2.69 | −3.71 | TF, PE, FB, M, LLD | ASD, cryptorchidism, MD | IC1-LOM | L (27.8) | N | N |
| 7 | Female | 38, 0 | −2.69 | −0.83 | 120 | ND | −1.01 | −0.81 | LLD | Normal | L (40.5) | N | N | |
| 8 | Male | 38, 0 | −3.55 | −1.86 | 17 | ND | −4.15 | −5.35 | M, FB | MD | Normal | L (29.0) | N | N |
| 9 | Female | 39, 4 | −2.01 | −1.37 | 135 | 72 | −1.24 | −1.26 | TF, PE, FB, M, LLD | Normal | L (30.0) | N | N | |
| 10 | Female | 38, 3 | −2.19 | −1.15 | 152 | 38 | −2.55 | −1.50 | PE, M, C | Normal | N | N | H (96.7) | |
| 11 | Female | 37, 6 | −2.11 | −0.48 | 91 | 43 | −2.14 | −2.33 | TF, PE, FB, M | Normal | N | N | H (97.2) | |
| 12 | Male | 38, 0 | −2.65 | −0.26 | 51 | ND | −2.38 | −3.60 | FB, C | Normal | N | N | N | |
| 13 | Female | 37, 1 | −3.34 | 0.39 | 63 | 15 | −0.21 | −1.75 | C, LLD | SUA, SD | IC1-LOM | L (19.5) | N | N |
| 14 | Male | 40, 0 | −2.77 | −0.35 | 50 | 16 | −2.17 | −3.13 | TF, PE, FB, M | Normal | N | N | N | |
| 15 | Female | 32, 4 | −2.46 | 1.36 | 52 | ND | −2.56 | −3.33 | PE, FB | SUA | Normal | N | N | N |
| 16 | Female | 38, 4 | −3.25 | −1.21 | 68 | ND | −2.77 | −4.21 | TF, PE, FB, M, C, LLD | MD | IC1-LOM | L (33.2) | N | N |
| 17 | Male | 39, 0 | −2.87 | 0.54 | 83 | ND | −5.42 | −8.10 | F | Normal | N | N | N | |
| 18 | Male | 38, 4 | −2.35 | −0.26 | 18 | ND | −2.51 | −1.46 | M | Normal | N | N | N | |
| 19 | Male | 40, 4 | −4.18 | 0.89 | 25 | ND | −4.30 | −5.35 | TF, PE, FB, M, C, | MD | IC1-LOM | L (12.1) | N | N |
| LLD, F | ||||||||||||||
| 20 | Female | 38, 1 | −2.85 | 0.49 | 10 | ND | −2.79 | −4.00 | TF, FB, F | VSD | Normal | N | N | N |
| 21 | Female | 38, 2 | −2.55 | −1.58 | 75 | ND | −6.31 | −6.58 | TF, PE, FB, C, F | Normal | N | N | H (96.9) | |
| 22 | Male | 35, 0 | −1.70 | 0.00 | 24 | ND | −1.84 | −2.44 | TF, PE, FB, M, LLD | MD | IC1-LOM | L (34.9) | N | N |
| 23 | Male | 34, 0 | −2.50 | −0.63 | 8 | ND | −4.22 | −5.59 | C, LLD | MD | IC1-LOM | L (26.1) | N | N |
| 24 | Male | 40, 0 | −2.09 | 0.19 | 15 | ND | −1.64 | −0.84 | TF, FB, M, C | IC1-LOM | L (35.6) | N | N | |
| 25 | Male | 35, 3 | −2.65 | −0.17 | 14 | ND | −4.26 | −5.61 | FB, M, F | VSD, cryptorchidism, MD | Normal | N | N | N |
| 26 | Male | 33, 2 | −2.27 | 1.49 | 118 | 36 | −5.93 | −4.07 | TF, PE, C, LLD, F | MD | Normal | N | N | N |
| 27 | Female | 35, 2 | −2.77 | 0.78 | 13 | ND | −3.12 | −5.28 | M, F | Cleft palate, ASD | IC1-LOM | L (32.7) | N | N |
| 28 | Male | 32, 5 | −1.84 | −0.35 | 12 | ND | −2.80 | −2.90 | TF, PE, FB, M, LLD | cryptorchidism | IC1-LOM | L (20.1) | N | N |
*Reference values (%mC, –2 SD to +2 SD) are as follows: IC1 (49.1–59.7), IC2 (56.4–69.9), MEST (47.8–54.3). Abbreviations: SDS, standard deviation score; MPH, midparental height; GH, growth hormone; SRS, Silver–Russell syndrome; MS-MLPA, methylation-specific multiplex ligation-dependent probe amplification; LOM, loss of methylation; ND, not done; PE, prominent ears; C, clinodactyly on the 5th finger; F, feeding difficulty; IC1: imprinting center 1; TF, triangular face; M, relative macrocephaly; LLD, leg length discrepancy; FB, frontal bossing; SD, speech delay; MD, motor delay; L, low; N, normal; H, high; ASD, atrial septal defect; SUA, single umbilical artery; VSD, ventricular septal defect.
Table 3
| Epigenetic variant (N = 17) | No epigenetic variant (N = 11) | P | |
|---|---|---|---|
| Birth weight (average SDS) | −2.68 | −2.41 | 0.505 |
| Gestational age [weeks on average (range)] | 37.8 (32.7–40.6) | 37.5 (32.6–40) | 0.551 |
| NH-CSS criteria [N, (%)] | |||
| Small for gestational age | 15 (88.2) | 11 (100.0) | 0.505 |
| Postnatal growth retardation | 14 (82.4) | 11 (100.0) | 0.258 |
| Relative macrocephaly | 13 (72.2) | 5 (45.5) | 0.097 |
| Frontal bossing | 12 (64.7) | 5 (45.5) | 0.441 |
| Body asymmetry | 11 (64.7) | 2 (18.2) | 0.024* |
| Feeding difficulties | 4 (23.5) | 5 (45.5) | 0.409 |
| Other manifestations [N, (%)] | |||
| Triangular face | 10 (58.8) | 4 (36.4) | 0.440 |
| Prominent ears | 9 (52.9) | 4 (36.4) | 0.460 |
| Clinodactyly on 5th fingers | 10 (58.8) | 4 (36.4) | 0.440 |
| Developmental delay [N, (%)] | 8 (47.1) | 2 (18.2) | 0.226 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download