1. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004; 328(7455):1519. PMID:
15213107.



3. Lim MK, Cho HJ. Current status of tobacco control policies in Korea compared with international treaty and its implementation. J Korean Med Assoc. 2018; 61(3):148–156.

4. Dent E, Lien C, Lim WS, Wong WC, Wong CH, Ng TP, et al. The Asia-Pacific clinical practice guidelines for the management of frailty. J Am Med Dir Assoc. 2017; 18(7):564–575. PMID:
28648901.


5. Kim KJ, Shin J, Choi J, Won CW. Discrepancies in the prevalence of known frailty scales: Korean Frailty and Aging Cohort Study. Ann Geriatr Med Res. 2018; 22(3):137–144. PMID:
32743263.



7. Xia Y, Bernert JT, Jain RB, Ashley DL, Pirkle JL. Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in smokers in the United States: NHANES 2007-2008. Biomarkers. 2011; 16(2):112–119. PMID:
21114376.


8. Goniewicz ML, Eisner MD, Lazcano-Ponce E, Zielinska-Danch W, Koszowski B, Sobczak A, et al. Comparison of urine cotinine and the tobacco-specific nitrosamine metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and their ratio to discriminate active from passive smoking. Nicotine Tob Res. 2011; 13(3):202–208. PMID:
21330276.



9. Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003; 12(11 Pt 1):1257–1261. PMID:
14652291.

10. Bernert JT, Pirkle JL, Xia Y, Jain RB, Ashley DL, Sampson EJ. Urine concentrations of a tobacco-specific nitrosamine carcinogen in the U.S. population from secondhand smoke exposure. Cancer Epidemiol Biomarkers Prev. 2010; 19(11):2969–2977. PMID:
20833972.


11. Yang HS, Lim H, Choi J, Bae S, Kim Y, Kwon HJ, et al. Environmental tobacco smoke exposure at home and attributable problem behaviors in Korean children and adolescents for 2012–2014 in a nationally representative survey. J Korean Med Sci. 2018; 33(36):e229. PMID:
30181731.

12. Ministry of Health and Welfare. The Physical Activity Guide for Koreans. Sejong: Ministry of Health and Welfare;2013.
13. Kalkbrenner AE, Hornung RW, Bernert JT, Hammond SK, Braun JM, Lanphear BP. Determinants of serum cotinine and hair cotinine as biomarkers of childhood secondhand smoke exposure. J Expo Sci Environ Epidemiol. 2010; 20(7):615–624. PMID:
20237497.



14. Ino T, Ohtani T, Yoshimi I. Urinary biomarkers for secondhand smoke. J Clin Lab Anal. 2011; 25(5):354–358. PMID:
21919071.



15. Vardavas CI, Tzatzarakis MN, Plada M, Tsatsakis AM, Papadaki A, Saris WH, et al. Biomarker evaluation of Greek adolescents' exposure to secondhand smoke. Hum Exp Toxicol. 2010; 29(6):459–466. PMID:
19939905.


16. Benowitz NL, Hukkanen J, Jacob P. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009; (192):29–60. PMID:
19184645.

17. Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005; 57(1):79–115. PMID:
15734728.


18. Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996; 18(2):188–204. PMID:
9021312.


19. International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon: IARC;2004. p. 1–1452.
20. Olivo-Marston SE, Yang P, Mechanic LE, Bowman ED, Pine SR, Loffredo CA, et al. Childhood exposure to secondhand smoke and functional mannose binding lectin polymorphisms are associated with increased lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2009; 18(12):3375–3383. PMID:
19959685.



21. Bjerregaard BK, Raaschou-Nielsen O, Sørensen M, Frederiksen K, Christensen J, Tjønneland A, et al. Tobacco smoke and bladder cancer--in the European prospective investigation into cancer and nutrition. Int J Cancer. 2006; 119(10):2412–2416. PMID:
16894557.


22. Torres S, Merino C, Paton B, Correig X, Ramírez N. Biomarkers of exposure to secondhand and thirdhand tobacco smoke: recent advances and future perspectives. Int J Environ Res Public Health. 2018; 15(12):2693.


23. Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999; 91(14):1194–1210. PMID:
10413421.


24. Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002; 23(6):907–922. PMID:
12082012.


25. Breyer-Pfaff U, Martin HJ, Ernst M, Maser E. Enantioselectivity of carbonyl reduction of 4-methylnitrosamino-1-(3-pyridyl)-1-butanone by tissue fractions from human and rat and by enzymes isolated from human liver. Drug Metab Dispos. 2004; 32(9):915–922. PMID:
15319331.

26. Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003; 3(10):733–744. PMID:
14570033.


27. Goniewicz ML, Havel CM, Peng MW, Jacob P 3rd, Dempsey D, Yu L, et al. Elimination kinetics of the tobacco-specific biomarker and lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Cancer Epidemiol Biomarkers Prev. 2009; 18(12):3421–3425. PMID:
19959691.



28. Singer BC, Hodgson AT, Guevarra KS, Hawley EL, Nazaroff WW. Gas-phase organics in environmental tobacco smoke. 1. Effects of smoking rate, ventilation, and furnishing level on emission factors. Environ Sci Technol. 2002; 36(5):846–853. PMID:
11918006.


29. Chao MR, Cooke MS, Kuo CY, Pan CH, Liu HH, Yang HJ, et al. Children are particularly vulnerable to environmental tobacco smoke exposure: evidence from biomarkers of tobacco-specific nitrosamines, and oxidative stress. Environ Int. 2018; 120:238–245. PMID:
30103123.


30. Lee HR, Kim HK, Yoo JS, Kim KN, Lee SY, Yoo SM, et al. Urine cotinine and environmental tobacco exposure in Korean adolescents. Korean J Fam Med. 2009; 30(1):31–38.

31. du Prel JB, Hommel G, Röhrig B, Blettner M. Confidence interval or p-value?: part 4 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2009; 106(19):335–339. PMID:
19547734.


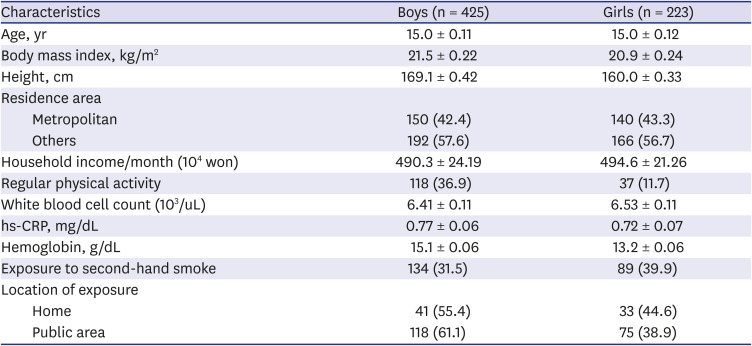
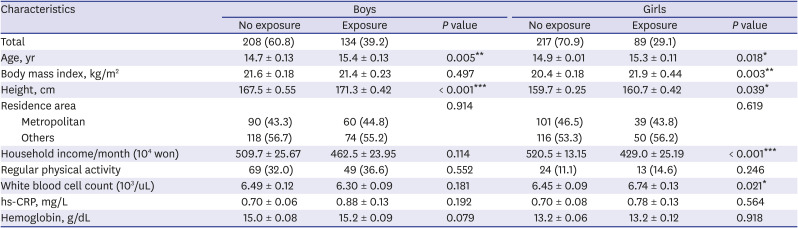
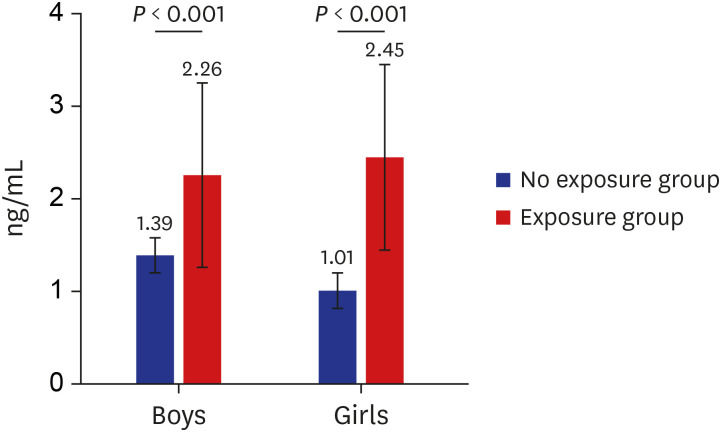
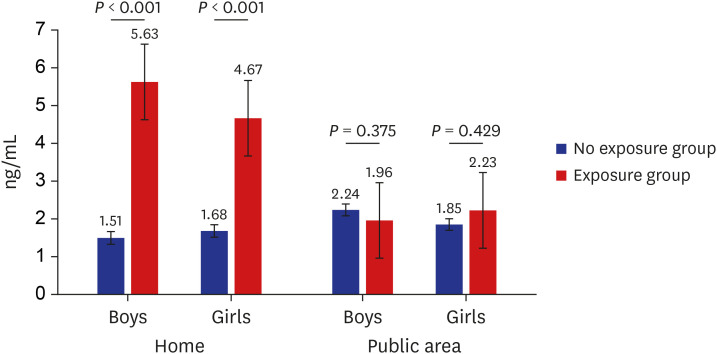




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download