1. El-Anwar MW, El-Aassar AS, El-Sayed H. Myringosclerosis in children with chronic renal failure on regular hemodialysis. Indian J Otol. 2015; 21(4):238–242.

2. Ozcan C, Görür K, Cinel L, Talas DU, Unal M, Cinel I. The inhibitory effect of topical N-acetylcysteine application on myringosclerosis in perforated rat tympanic membrane. Int J Pediatr Otorhinolaryngol. 2002; 63(3):179–184. PMID:
11997152.
3. Selcuk A, Ensari S, Sargin AK, Can B, Dere H. Histopathological classification of tympanosclerotic plaques. Eur Arch Otorhinolaryngol. 2008; 265(4):409–413. PMID:
17962967.

4. Gibb AG, Pang YT. Current considerations in the etiology and diagnosis of tympanosclerosis. Eur Arch Otorhinolaryngol. 1994; 251(8):439–451. PMID:
7718216.

5. Uneri C, Bağlam T, Yazici M. The effect of vitamin E treatment on the development of myringosclerosis after ventilation tube insertion. Int J Pediatr Otorhinolaryngol. 2006; 70(6):1045–1048. PMID:
16368152.

6. Khodaverdi M, Jørgensen G, Lange T, Stangerup SE, Drozdziewizc D, Tos M, et al. Hearing 25 years after surgical treatment of otitis media with effusion in early childhood. Int J Pediatr Otorhinolaryngol. 2013; 77(2):241–247. PMID:
23218983.

7. Yaman H, Guclu E, Yilmaz S, Ozturk O. Myringosclerosis after tympanostomy tube insertion: relation with tube retention time and gender. Auris Nasus Larynx. 2010; 37(6):676–679. PMID:
20392579.

8. Asiri S, Hasham A, al Anazy F, Zakzouk S, Banjar A. Tympanosclerosis: review of literature and incidence among patients with middle-ear infection. J Laryngol Otol. 1999; 113(12):1076–1080. PMID:
10767919.

9. Bhaya MH, Schachern PA, Morizono T, Paparella MM. Pathogenesis of tympanosclerosis. Otolaryngol Head Neck Surg. 1993; 109(3 Pt 1):413–420. PMID:
8414556.

10. Kim BJ, Kim J, Park IY, Jung JY, Suh MW, Oh SH. Effects of transient auditory deprivation during critical periods on the development of auditory temporal processing. Int J Pediatr Otorhinolaryngol. 2018; 104:66–71. PMID:
29287884.

11. Branco C, Monteiro D, Paço J. Predictive factors for the appearance of myringosclerosis after myringotomy with ventilation tube placement: randomized study. Eur Arch Otorhinolaryngol. 2017; 274(1):79–84. PMID:
27395069.

12. Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg. 2001; 124(4):374–380. PMID:
11283489.

13. Luntz M, Sadé J. Growth of the eustachian tube lumen with age. Am J Otolaryngol. 1988; 9(5):195–198. PMID:
3228177.

14. Takasaki K, Sando I, Balaban CD, Haginomori S, Ishijima K, Kitagawa M. Histopathological changes of the eustachian tube cartilage and the tensor veli palatini muscle with aging. Laryngoscope. 1999; 109(10):1679–1683. PMID:
10522942.

15. In : Casselbrant ML, Brostoff LM, Cantekin EI, Ashoff VM, Bluestone CD, editors. Otitis media in children in the United States. Proceedings of the International Conference on Acute and Secretory Otitis Media; 1985 Nov 17-22; Jerusalem, Israel. Amsterdam: Kugler Publication;1986. p. 161–164.
16. Bylander A, Tjernström O. Changes in Eustachian tube function with age in children with normal ears. A longitudinal study. Acta Otolaryngol. 1983; 96(5-6):467–477. PMID:
6637457.
17. Bluestone CD. Eustachian Tube: Structure, Function, and Role in Otitis Media. Hamilton: BC Decker Inc.;2005.
18. Akyıldız N. Kulak hastalıkları ve mikrocerrahisi. In : Akyıldız N, editor. Kulak Hastalıkları ve Mikrocerrahisi 1. Ankara: Bilimsel Tıp Yayınevi;1998. p. 461–472.
19. Mattsson C, Marklund SL, Hellström S. Application of oxygen free radical scavengers to diminish the occurrence of myringosclerosis. Ann Otol Rhinol Laryngol. 1997; 106(6):513–518. PMID:
9199613.

20. Karlidağ T, Ilhan N, Kaygusuz I, Keleş E, Yalçin S. Comparison of free radicals and antioxidant enzymes in chronic otitis media with and without tympanosclerosis. Laryngoscope. 2004; 114(1):85–89. PMID:
14710000.
21. Branco C, Paço J. Does calcemia influence the onset of myringosclerosis after myringotomy with the insertion of ventilation tubes? Acta Otorrinolaringol Esp. 2017; 68(6):323–327. PMID:
28522133.

22. Park YH, Park CH, Kim HJ. The effect of topical sodium thiosulfate in experimentally induced myringosclerosis. Laryngoscope. 2010; 120(7):1405–1410. PMID:
20583241.

23. Akdagli S, Tuzuner A, Demirci S, Karadas H, Tulaci KG, Dogan M, et al. Do all antioxidant supplements have the same potential effect on preventing myringosclerosis? Clin Exp Otorhinolaryngol. 2015; 8(1):1–6. PMID:
25729488.

24. Arslan N, Tepe D, Taştan E, Demirci M, Caydere M, Ustun H, et al. Evaluation of the effectiveness of topical ciprofloxacin and prednisolone in the prevention of myringosclerosis. Eur Arch Otorhinolaryngol. 2012; 269(11):2335–2341. PMID:
22197890.

25. Erdurak SC, Coskun BU, Sakalli E, Tansuker HD, Turan F, Kaya D. Does the use of radiofrequency myringotomy for insertion of a ventilation tube reduce the incidence of myringosclerosis? Eur Arch Otorhinolaryngol. 2014; 271(3):459–462. PMID:
23494285.

26. Koc A, Uneri C. Sex distribution in children with tympanosclerosis after insertion of a tympanostomy tube. Eur Arch Otorhinolaryngol. 2001; 258(1):16–19. PMID:
11271428.

27. Koç A, Uneri C. Genetic predisposition for tympanosclerotic degeneration. Eur Arch Otorhinolaryngol. 2002; 259(4):180–183. PMID:
12064505.

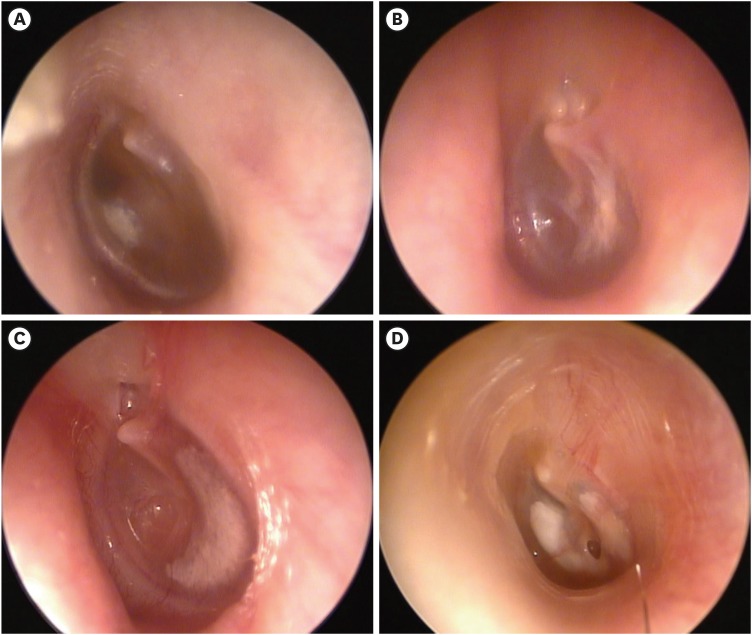
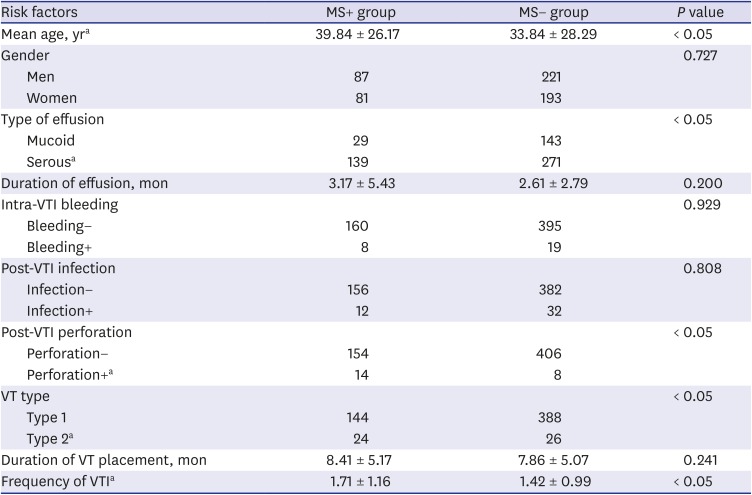
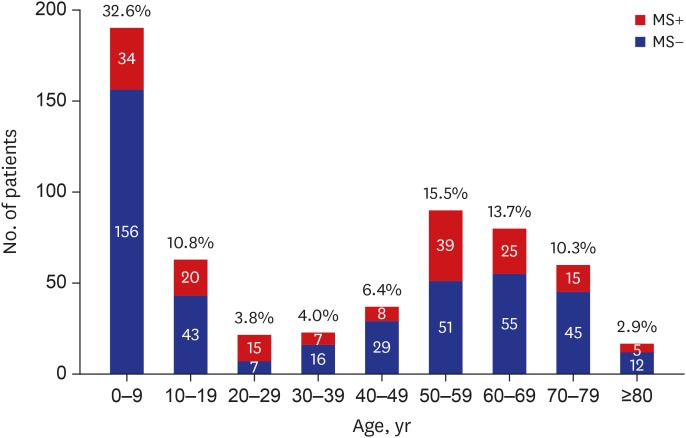
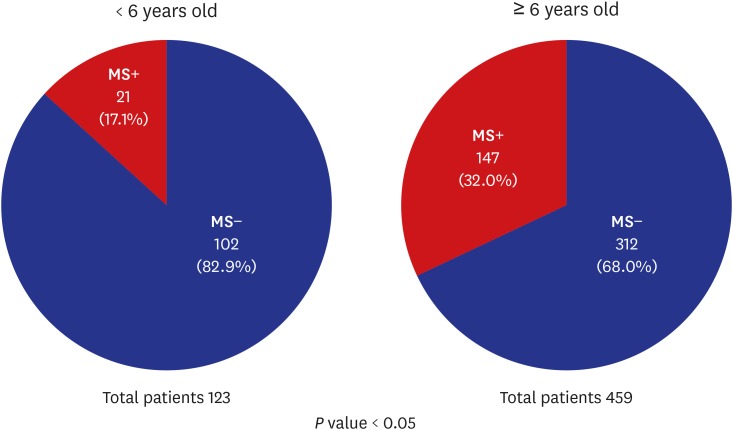




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download