This article has been
cited by other articles in ScienceCentral.
Abstract
The neurologic manifestations concerning coronavirus disease 2019 (COVID-19) are highly penetrated. Anosmia and ageusia are one of the common acute neurologic symptoms, which develop in the early stage of COVID-19. However, it is not reported that how immunosuppressive agents affect these symptoms. We report olfactory and gustatory dysfunctions in a patient with ankylosing spondylitis (AS) treated with etanercept during COVID-19. A 53-year-old female showing AS controlled with tumor necrosis factor-α inhibitor, etanercept, had been diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, presenting cough and rhinorrhea. One month after diagnosis, she complained about hyposmia and hypogeusia two days before the seronegative conversion of SARS-CoV-2, which were confirmed by a neurological examination. We speculate that the etanercept may have delayed the development of olfactory and gustatory dysfunction in the patient.
Keywords: Severe Acute Respiratory Syndrome Coronavirus 2, Tumor Necrosis Factor-alpha, Neurologic Manifestations
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic outbreak that typically presents with fever, cough, dyspnea, and fatigue. Moreover, patients with COVID-19 were recently reported to have atypical neurologic manifestations such as hyposmia and hypogeusia.
1234 In general, patients on immunomodulatory treatments, including tumor necrosis factor (TNF)-α inhibitors considered as a particularly vulnerable group with an increased risk of infections.
5 Appropriate prevention measures should be followed to reduce the risk of infection among patients treated with TNF-α inhibitors.
6 Fortunately, several reports speculated that patients on TNF-α inhibitors do not seem to be associated with a severe evolution of the COVID-19.
78 However, the neurological symptoms of COVID-19 in rheumatic disease patients taking TNF-α inhibitors are unknown, and objective neurologic examinations for patients with COVID-19 have rarely been reported.
CASE DESCRIPTION
We report a case of olfactory and gustatory dysfunction in a 53-year-old female patient with ankylosing spondylitis (AS) treated with a TNF-α inhibitor, etanercept, during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. She was diagnosed with AS as human leukocyte antigen B-27 positivity, bilateral sacroiliitis, enthesitis, and C-reactive protein (CRP) elevation in March 2017. Although she received multiple nonsteroidal anti-inflammatory drugs (NSAIDs) and disease-modifying anti-rheumatic drugs (sulfasalazine 2,000 mg per every day and methotrexate 15 mg per every week), her symptoms waxed and waned. Treatment with subcutaneous etanercept 50 mg once weekly was initiated, which led to good control with normal CRP from November 2018. Then, NSAIDs and sulfasalazine were discontinued, but methotrexate was retained. At the last assessment in December 2019, her symptoms remained improved, so after that, she received etanercept at 3-week intervals.
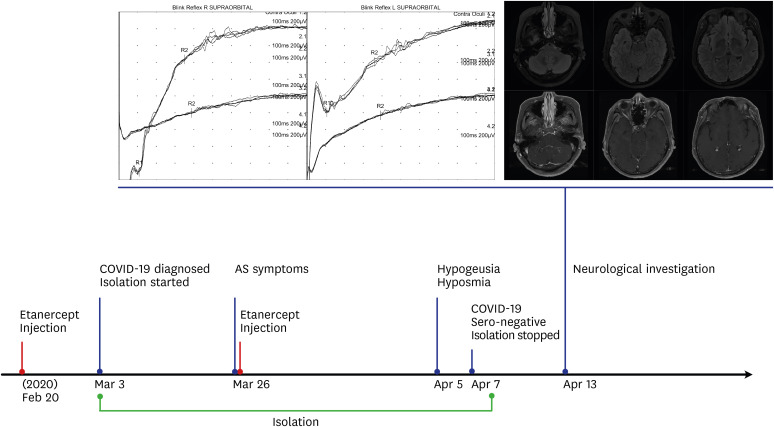
After contact with a patient with SARS-CoV-2, she was diagnosed with COVID-19 on March 3, 2020, and the last etanercept injection was administered on February 20. Her symptoms were mild (i.e., cough and rhinorrhea but no fever) without tasty or gustatory abnormality, and she was isolated on March 3. On March 25, she had AS symptoms and self-administered etanercept. After two days of SARS-CoV-2 negative test results on April 6 and 7, she was released from isolation. However, she had recognized a decreased sensation of taste, including sweet, salty, and sour taste on April 5 (
Fig. 1). She was transferred to a neurologist for an objective examination. On neurological examination, she was able to perceive the smell of ground coffee beans, but moderately decreased smell intensity and severely disturbed sweet taste were noticed after 50% dextrose water was orally administered. Her other cranial nerves were normal; namely, extraocular movement, facial muscle expression, somatic sensation of the tongue, hearing, and gag reflex were normal. The electrophysiologic studies of facial nerve conduction and blink reflex were normal (
Fig. 1). A brain magnetic resonance imaging showed no abnormalities (
Fig. 1).
Fig. 1
The timeline of clinical data, results of the blink reflex, and brain MRI. Clinical presentation and etanercept administration are depicted on the appropriate date. The blink reflex showed normal R1 and R2 responses bilaterally. A brain MRI revealed normal structures, including a normal frontal lobe, maxilla, sphenoid, and frontal sinus. The patient consented to publish her clinical records and images.
COVID-19 = coronavirus disease 2019, MRI = magnetic resonance imaging, AS = ankylosing spondylitis.
Ethics statement
Written informed consent for publication concerning all photographic materials was received.
DISCUSSION
After we performed a neurologic investigation, we confirmed that the patient only had olfactory and gustatory sensory dysfunction. In line with a previous result, our findings do not suggest the patient was at a higher risk of life-threatening complications from COVID-19 compared to the general population.
78 However, it is possible that a TNF-α inhibitor treatment during the SARS-CoV-2 infection delayed the development of olfactory and gustatory dysfunction in the patient, unlike the circumstances in a previous report that demonstrated neurologic manifestations occurring early in the illness.
12349
Although the prevalence of olfactory and gustatory sensory dysfunction in COVID-19 patients depends on the variation among study samples,
12349 a large telephone-based study demonstrated that these symptoms were 15.1% of total patients and higher in female and young age under 50.
9 Interestingly, anosmia and ageusia are not accompanied by nasal or oral symptoms, which developed acutely within four days of dyspnea or febrile complaints,
49 and these neurologic dysfunctions are mostly temporary, showing recovery period within three weeks.
9 These are probably related to the direct invasion of the virus on the olfactory and gustatory receptors.
Hypotheses for pathologic mechanisms of olfactory and gustatory dysfunction during COVID-19 infection are not clear. However, angiotensin-converting enzyme 2 (ACE2) receptors seem to have a key role.
10 Expression of ACE2 receptors is relatively higher in oral tongue mucosa and surfactant-producing type 2 pneumocytes than in other organs.
1011 SARS-CoV-2 invades ACE2 receptors for cellular entry, and then inflammation can be introduced in these tissues.
11 Damage of mucosal epithelium of tongue can explain ageusia, and a similar mechanism can be applied to anosmia.
91011
In general, the interruption of TNF-α inhibitors used in rheumatic diseases is not recommended because it may be associated with the onset of clinical flares with the subsequent addition of other immunosuppressants.
12 Although TNF-α inhibitors should be discontinued during serious infections, etanercept was reported to have a protective effect on olfactory inflammation in a mouse model.
513 Etanercept treatment in inducible olfactory inflammation mouse showed functional and histological recovery by inhibiting TNF-α induced inflammatory cascade.
13
In conclusion, we report olfactory and gustatory dysfunction in a patent with AS treated with etanercept during COVID-19. Whether this agent positively or negatively affects the course of COVID-19 in humans, especially the development of olfactory and gustatory dysfunction, is uncertain until further and larger studies can be performed.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download