1. Brożek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, Brignardello-Petersen R, Canonica GW, Casale T, Chavannes NH, Correia de Sousa J, Cruz AA, Cuello-Garcia CA, Demoly P, Dykewicz M, Etxeandia-Ikobaltzeta I, Florez ID, Fokkens W, Fonseca J, Hellings PW, et al. 2017; Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 140:950–958. DOI:
10.1016/j.jaci.2017.03.050. PMID:
28602936.

2. D'Amato G, Pawankar R, Vitale C, Lanza M, Molino A, Stanziola A, Sanduzzi A, Vatrella A, D'Amato M. 2016; Climate change and air pollution: effects on respiratory allergy. Allergy Asthma Immunol Res. 8:391–395. DOI:
10.4168/aair.2016.8.5.391. PMID:
27334776. PMCID:
PMC4921692.
3. Ricketti PA, Alandijani S, Lin CH, Casale TB. 2017; Investigational new drugs for allergic rhinitis. Expert Opin Investig Drugs. 26:279–292. DOI:
10.1080/13543784.2017.1290079. PMID:
28141955.

5. Greiner AN, Meltzer EO. 2006; Pharmacologic rationale for treating allergic and nonallergic rhinitis. J Allergy Clin Immunol. 118:985–998. DOI:
10.1016/j.jaci.2006.06.029. PMID:
17088121.

7. Dantzer JA, Wood RA. 2018; The use of omalizumab in allergen immunotherapy. Clin Exp Allergy. 48:232–240. DOI:
10.1111/cea.13084. PMID:
29315922.

8. Long R, Zhou Y, Huang J, Peng L, Meng L, Zhu S, Li J. 2015; Bencycloquidium bromide inhibits nasal hypersecretion in a rat model of allergic rhinitis. Inflamm Res. 64:213–223. DOI:
10.1007/s00011-015-0800-6. PMID:
25690567.

10. Rogers DF. 2003; Airway hypersecretion in allergic rhinitis and asthma: new pharmacotherapy. Curr Allergy Asthma Rep. 3:238–248. DOI:
10.1007/s11882-003-0046-1. PMID:
12662474.

11. Kremer B, den Hartog HM, Jolles J. 2002; Relationship between allergic rhinitis, disturbed cognitive functions and psychological well-being. Clin Exp Allergy. 32:1310–1315. DOI:
10.1046/j.1365-2745.2002.01483.x. PMID:
12220469.

12. Galietta LJ, Pagesy P, Folli C, Caci E, Romio L, Costes B, Nicolis E, Cabrini G, Goossens M, Ravazzolo R, Zegarra-Moran O. 2002; IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J Immunol. 168:839–845. DOI:
10.4049/jimmunol.168.2.839. PMID:
11777980.

13. Zhou Y, Shapiro M, Dong Q, Louahed J, Weiss C, Wan S, Chen Q, Dragwa C, Savio D, Huang M, Fuller C, Tomer Y, Nicolaides NC, McLane M, Levitt RC. 2002; A calcium-activated chloride channel blocker inhibits goblet cell metaplasia and mucus overproduction. Novartis Found Symp. 248:150–165. discussion 165–170. 277–282. DOI:
10.1002/0470860790.ch10. PMID:
12568493.

14. Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. 2008; TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 322:590–594. DOI:
10.1126/science.1163518. PMID:
18772398.

15. Cho HJ, Choi JY, Yang YM, Hong JH, Kim CH, Gee HY, Lee HJ, Shin DM, Yoon JH. 2010; House dust mite extract activates apical Cl
- channels through protease-activated receptor 2 in human airway epithelia. J Cell Biochem. 109:1254–1263. DOI:
10.1002/jcb.22511. PMID:
20186875.
16. Rievaj J, Davidson C, Nadeem A, Hollenberg M, Duszyk M, Vliagoftis H. 2012; Allergic sensitization enhances anion current responsiveness of murine trachea to PAR-2 activation. Pflugers Arch. 463:497–509. DOI:
10.1007/s00424-011-1064-9. PMID:
22170096.

17. Kang JW, Lee YH, Kang MJ, Lee HJ, Oh R, Min HJ, Namkung W, Choi JY, Lee SN, Kim CH, Yoon JH, Cho HJ. 2017; Synergistic mucus secretion by histamine and IL-4 through TMEM16A in airway epithelium. Am J Physiol Lung Cell Mol Physiol. 313:L466–L476. DOI:
10.1152/ajplung.00103.2017. PMID:
28546154.

18. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY, Rock JR. 2012; Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 109:16354–16359. DOI:
10.1073/pnas.1214596109. PMID:
22988107. PMCID:
PMC3479591.

19. Scudieri P, Caci E, Bruno S, Ferrera L, Schiavon M, Sondo E, Tomati V, Gianotti A, Zegarra-Moran O, Pedemonte N, Rea F, Ravazzolo R, Galietta LJ. 2012; Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol. 590:6141–6455. DOI:
10.1113/jphysiol.2012.240838. PMID:
22988141. PMCID:
PMC3530122.

20. Qiao X, He WN, Xiang C, Han J, Wu LJ, Guo DA, Ye M. 2011; Qualitative and quantitative analyses of flavonoids in Spirodela polyrrhiza by high-performance liquid chromatography coupled with mass spectrometry. Phytochem Anal. 22:475–483. DOI:
10.1002/pca.1303. PMID:
21465598.

21. Lee HJ, Kim MH, Choi YY, Kim EH, Hong J, Kim K, Yang WM. 2016; Improvement of atopic dermatitis with topical application of Spirodela polyrhiza. J Ethnopharmacol. 180:12–17. DOI:
10.1016/j.jep.2016.01.010. PMID:
26778605.

22. Nam JH, Jung HW, Chin YW, Yang WM, Bae HS, Kim WK. 2017; Spirodela polyrhiza extract modulates the activation of atopic dermatitis-related ion channels, Orai1 and TRPV3, and inhibits mast cell degranulation. Pharm Biol. 55:1324–1329. DOI:
10.1080/13880209.2017.1300819. PMID:
28290212. PMCID:
PMC6130684.

24. Kircher S, Merino-Wong M, Niemeyer BA, Alansary D. 2018; Profiling calcium signals of in vitro polarized human effector CD4+ T cells. Biochim Biophys Acta Mol Cell Res. 1865:932–943. DOI:
10.1016/j.bbamcr.2018.04.001. PMID:
29626493.
25. Park BK, Park YC, Jung IC, Kim SH, Choi JJ, Do M, Kim SY, Jin M. 2015; Gamisasangja-tang suppresses pruritus and atopic skin inflammation in the NC/Nga murine model of atopic dermatitis. J Ethnopharmacol. 165:54–60. DOI:
10.1016/j.jep.2015.02.040. PMID:
25721805.

26. Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. 2008; TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 455:1210–1215. DOI:
10.1038/nature07313. PMID:
18724360.

27. Namkung W, Phuan PW, Verkman AS. 2011; TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 286:2365–2374. DOI:
10.1074/jbc.M110.175109. PMID:
21084298. PMCID:
PMC3023530.

28. Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. 2009; Bestrophin and TMEM16-Ca
2+ activated Cl
- channels with different functions. Cell Calcium. 46:233–241. DOI:
10.1016/j.ceca.2009.09.003. PMID:
19783045.
29. Fischer H, Illek B, Sachs L, Finkbeiner WE, Widdicombe JH. 2010; CFTR and calcium-activated chloride channels in primary cultures of human airway gland cells of serous or mucous phenotype. Am J Physiol Lung Cell Mol Physiol. 299:L585–L594. DOI:
10.1152/ajplung.00421.2009. PMID:
20675434. PMCID:
PMC2957417.

30. Banga A, Flaig S, Lewis S, Winfree S, Blazer-Yost BL. 2014; Epinephrine stimulation of anion secretion in the Calu-3 serous cell model. Am J Physiol Lung Cell Mol Physiol. 306:L937–L946. DOI:
10.1152/ajplung.00190.2013. PMID:
24705724. PMCID:
PMC4025061.

31. Seo Y, Lee HK, Park J, Jeon DK, Jo S, Jo M, Namkung W. 2016; Ani9, a novel potent small-molecule ANO1 inhibitor with negligible effect on ANO2. PLoS One. 11:e0155771. DOI:
10.1371/journal.pone.0155771. PMID:
27219012. PMCID:
PMC4878759.

32. Seo Y, Ryu K, Park J, Jeon DK, Jo S, Lee HK, Namkung W. 2017; Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS One. 12:e0174935. DOI:
10.1371/journal.pone.0174935. PMID:
28362855. PMCID:
PMC5376326.

33. Zhuo RG, Peng P, Zheng JQ, Zhang YL, Wen L, Wei XL, Ma XY. 2017; The glycine hinge of transmembrane segment 2 modulates the subcellular localization and gating properties in TREK channels. Biochem Biophys Res Commun. 490:1125–1131. DOI:
10.1016/j.bbrc.2017.06.200. PMID:
28676394.

34. Devor DC, Singh AK, Lambert LC, DeLuca A, Frizzell RA, Bridges RJ. 1999; Bicarbonate and chloride secretion in Calu-3 human airway epithelial cells. J Gen Physiol. 113:743–760. DOI:
10.1085/jgp.113.5.743. PMID:
10228185. PMCID:
PMC2222914.

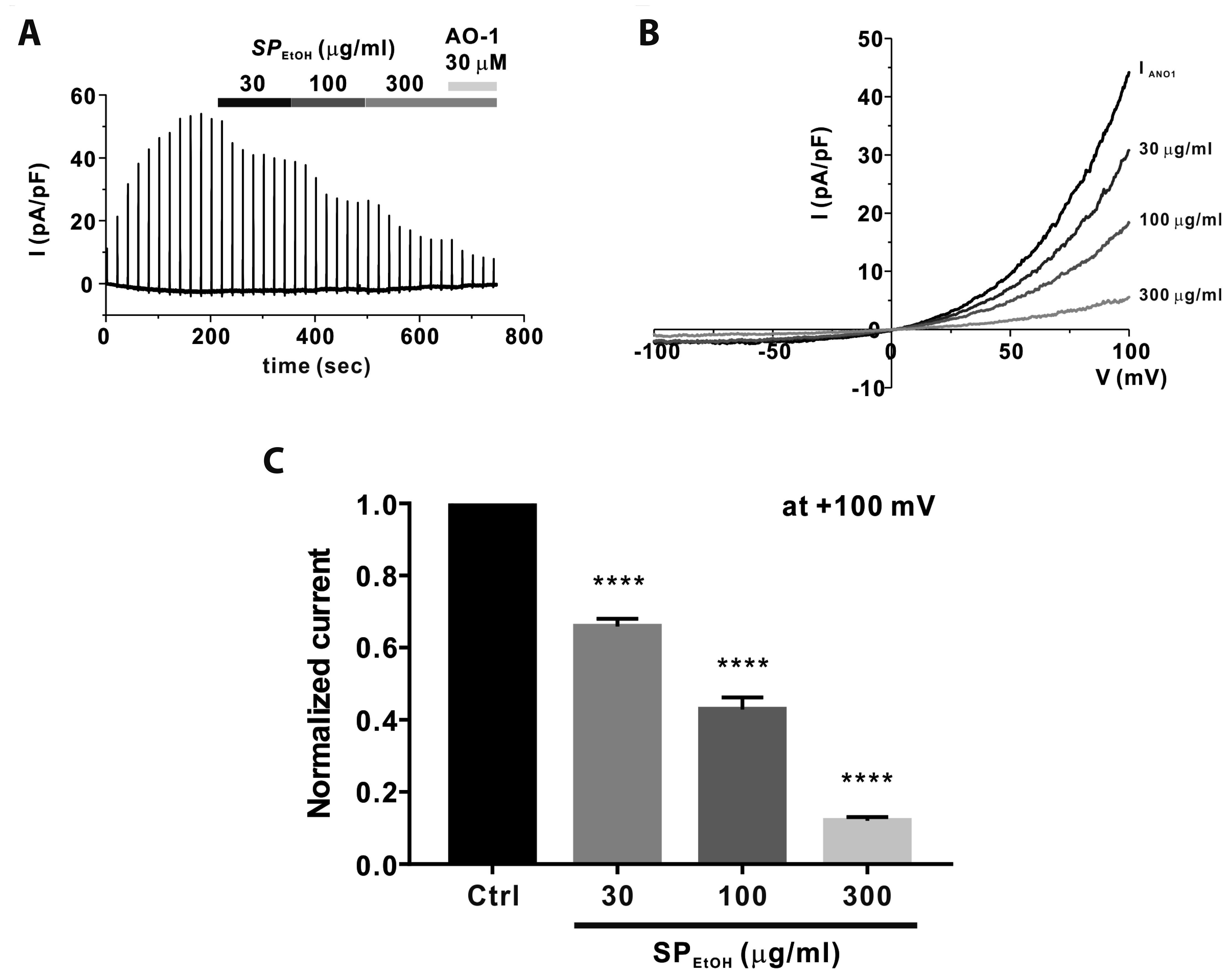
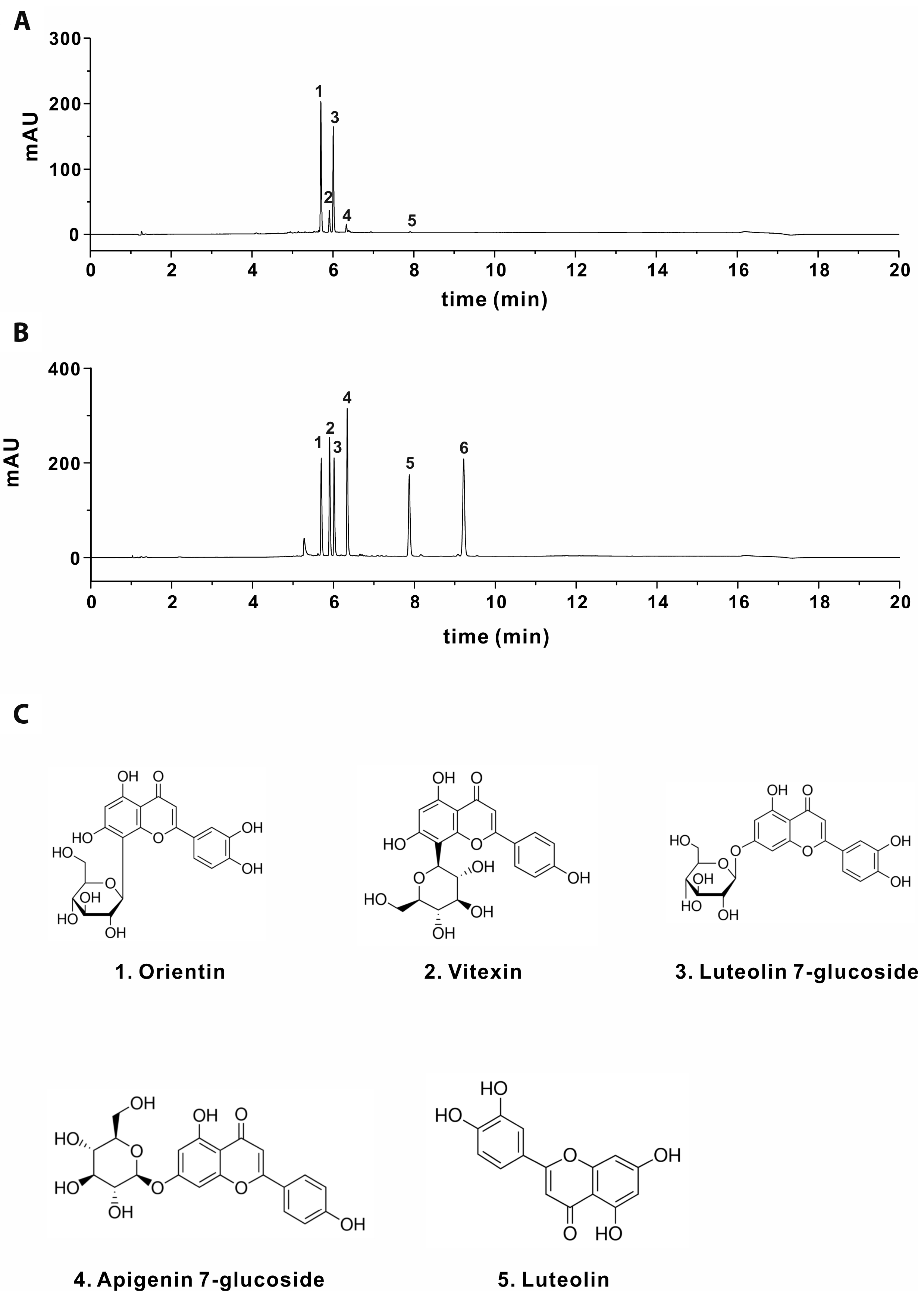
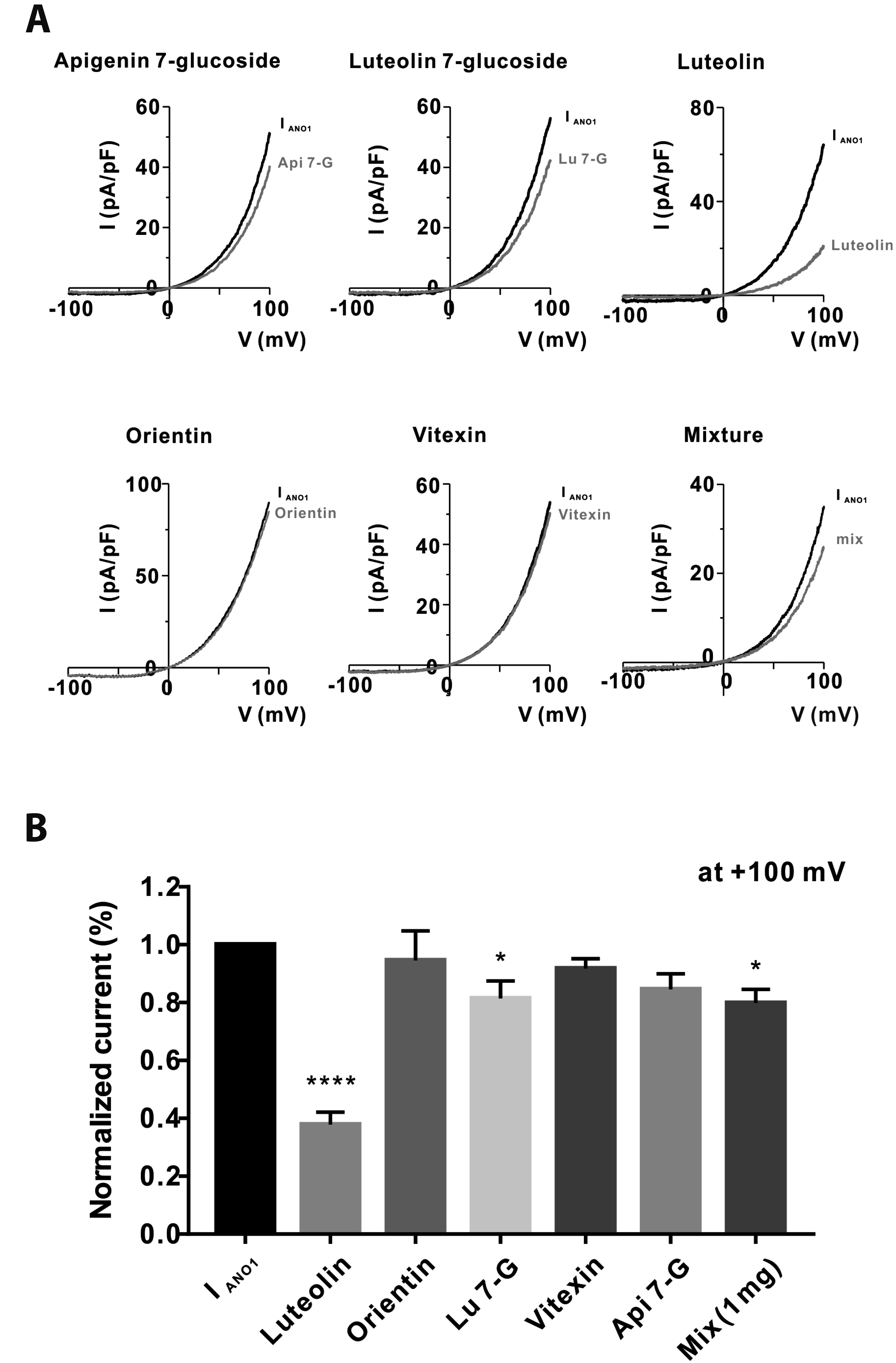
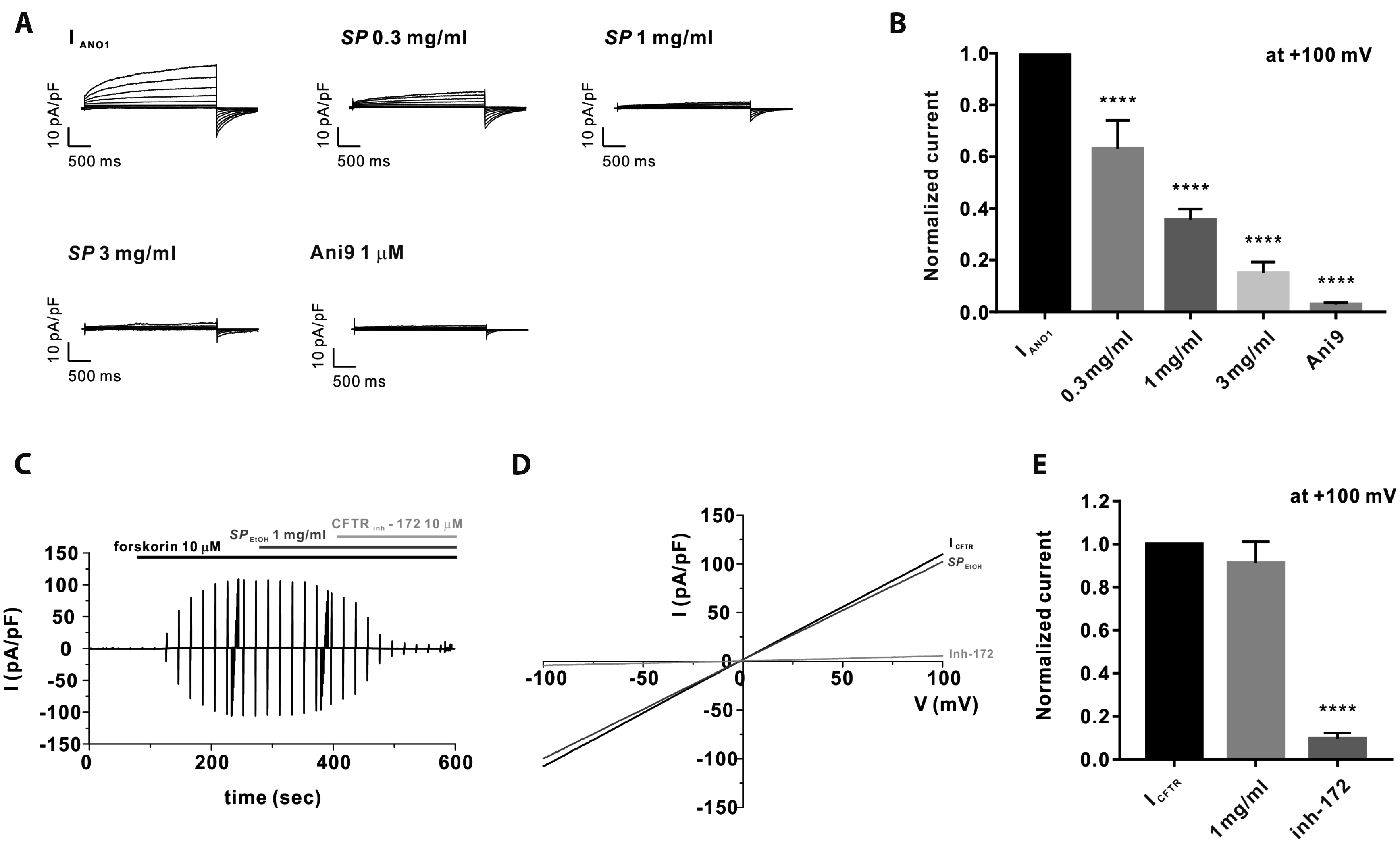




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download