Abstract
Cardiovascular diseases are the primary reason of mortality, among which myocardial infarction (MI) is the most dominant and prevalent. This study was considered to examine D-Limonene protective action against isoproterenol (ISO) induced MI. Wister male rats were dispersed into four groups. Normal and D-Limonene control group in which rats administered saline or D-Limonene. ISO control animals were administered saline for 21 days then challenged with ISO (85 mg/kg, subcutaneously) on 20th and 21st day for MI induction. D-Limonene pretreated group in which animals were pretreated with D-Limonene 50 mg/kg orally for 21 days then administered ISO on 20th and 21st day. MI prompted variations were assessed by myocardial infarction area determination, blood pressure (BP) alterations, cardiac injury biomarkers and inflammatory mediators measurements. For more depth investigation, both the apoptotic status was evaluated via measuring mRNA expression of Bcl-2 and Bax as well as mitogen-activated protein kinase-extracellular signal-regulated kinase (MAPK-ERK) signal transduction were investigated via Western blotting. MI group revealed significant infarcted area, blood pressure alterations, myocardial injury enzymes intensification together with inflammatory cytokines amplification. MI was associated with activation of MAPK-ERK signal pathway and apoptotic status within the myocardium. On the other hand, pretreated with D-Limonene demonstrated deterred infracted area, reduced myocardial enzymes, improved BP indices, lessened inflammatory levels. Furthermore, D-Limonene pretreatment caused a decline in MAPK proteins pathway and Bax relative mRNA expression, while intensifying Bcl-2 mRNA expression promoting that D-Limonene may constrain MI induced myocardial apoptosis. D-Limonene mitigated MI injury through MAPK/NF-κB pathway inhibition and anti-apoptotic effect.
D-Limonene, monocyclic monoterpene, is a main component in numerous citrus oils (orange, lemon, mandarin, lime, and grapefruit) [1]. D-Limonene demonstrated various pharmacological actions include anti-inflammatory [2-7], antioxidant [7-11], lipid-lowering [11], anti-atherogenic [11], anti-nociceptive [12], anti-diabetic [13] anti-fibrotic [14], chemo-preventive [1] and immunosuppressive [15] actions. Also D-Limonene managed metabolic syndrome [16,17], and decreased of serum lipid levels via modulating liver X receptors signaling [16]. In addition, D-Limonene exerted healing effects in the gastrointestinal tract [6,7,18], relieving occasional heartburn and gastroesophageal reflux disorder therefore; has been clinically used to dissolve cholesterol-containing gallstones [1]. As for the anti-inflammatory effects, D-Limonene demonstrated anti-inflammatory effect specifically through reducing the levels of tumor necrosis factor (TNF)-α [2,3,6,12], interleukin (IL)-6 [2], IL-1β [2,12] and NF-κB [2,4,6].
Among all cardiovascular diseases, myocardial infarction (MI) is the most dominant and prevalent [19]. Disproportion between coronary blood required and supply to the heart results in MI [20]. Isoproterenol (ISO), β adrenergic agonist, in high doses, can upsurges myocardial burden, triggering myocardial dysfunction in animals mimicking the pathological and morphological deviations happening in humans. Augmented free radicals production during MI causes stimulation of mitogen activated protein kinase (MAPK) signaling pathway [21].
MAPK involve three subfamilies; Extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun-N-terminal kinase (JNK) and p38. MAPK signaling pathway promote NF-κB, which provokes additional inflammatory cytokines triggering extra injury within the myocardium tissue [21]. ERK signaling pathway, the most studied, is involved in regulating multiple biological processes, such as survival, growth and death of various cells and inflammation-related immune response [22]. While JNK and p38 MAPK prompt apoptosis, ERK stimulates cell existence and is found to be decreased in MI.
The objective of this study was to investigate the underlying mechanism of Limonene in the attenuation of myocardial ischemia injury was interrelated to the MAPK/ERK/NF-κB pathway inhibition.
Wister male rats (220–250 g) were kept in typical laboratory environment, allowed all the time to chow pellet nutrition and tap water ad libitum. Animal experimental practices and techniques entirely were applied in consistent with the Ethical Conduct for the Animals Use in Research. Recomendations and only after the permission from the Animal Research Ethics Committee at King Faisal University (KFU-REC/2019-2-4).
Rats were distributed into 4 groups haphazardly (n = 6). Normal group in which rats administered saline for 21 days orally then saline subcutaneously (s.c.) on 20th and 21st day. Limonene control group in which the animals were managed with D-Limonene purchased from Sigma Company, (50 mg/kg, orally) [7,12] for 21 days and then injected with saline (s.c.) on 20th and 21st day day. ISO control group in which animals were administered saline orally for 21 days then administered ISO (85 mg/kg, s.c.) [19,23] on 20th and 21st day for myocardial infraction induction. D-Limonene pretreatment group in which animals were treated with D-Limonene 50 mg/kg orally for 21 days then received ISO (85 mg/kg, s.c.) on 20th and 21st day. All experimental animals were sacrificed 24 h after second dose of ISO.
Following BP measurements, blood samples from diferent eperimental groups were collected from the left ventricle, centrifuged (20 min/4,000 rpm) and the detached serum was kept in −80°C deep freezer, for the subsequent biochemical investigations. Animals' hearts were rapidly separated, washed with saline, weighed and freezed (−20°C) as mentioned in earlier report [26].
Frozen hearts were cut into four to five sections and immersed in 1% Triphenyl Tetrazolium Chloride (TTC) solution (37°C, 20 min, dark room). Myocardial infarction area was measured and expressed as percent of infarct size was measured by Image J software (http://imagej.net) as mentioned before [27].
Myocardial enzymes are impeartive pointers of myocardial cells inevitability. Cardiac tissue homogenate obtained from different experimental groups were used to measure Creatine Phosphokinase (CPK), Creatine Kinase Myocardial Bound (CK-MB), Cardiac tropinin T (cTnT), and Cardiac tropinin I (cTnI) using ELISA kits.
The acquired suerm was used to detect different inflammtory mediators levels such as TNF-α, IL-6, IL-1β, and NF-κB using ELISA kits according to the manufacturer's directions (R&D Systems, Minneapolis, MN, USA).
RNA was extracted consuming TRIzol following to the manufacturer's directions (Invitrogen, Carlsbad, CA, USA). cDNA was created using Superscript II reverse transcriptase (Invitrogen) then amplified via SYBR green PCR master mix (Applied Biosystems, Foster City, CA, USA). The samples were analyzed in duplicate, and β actin was used as house keeping gene. The Ct values were measured by 7900 HT Real-Time PCR System (Applied Biosystems) and the fold changes of gene expression were considered by the 2−ΔΔCt method [2]. Primer sequences used in this study were as follows: Bcl-2-F: 5’-CCGGG AGATCGTGATGAAGT-3’, Bcl-2-R: 5’-ATCCCAGCCTCCGTTATCCT-3’, Bax-F: 5’-GTGGTTGCCCTCTTCTACTTTG-3’, Bax-R: 5’-CACAAAGATGGTCACTGTC TGC-3’, β-actin-F: 5’-TGACAGGATGCAGAAGGAGA-3’, β-actin-R: 5’-TAGAGCC ACCAATCCACACA-3’.
Forzen hearts were homogenised in RIPA buffer containing protease inhibitor, centrifuged (10,000 rpm, 10 min, 4°C), and the protein content in the gained supernatant was measured by NanoDropLitespectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Protein samples (45 µg) were electrophoresed in SDS-PAGE, transported to PDVF membrane, incubated with 5% BSA first and then with primary antibodies of ERK1/2, p-ERK1/2, JNK, p-JNK, p38, P-p38 and GAPDH (1:1,000). These primary antibodies were identified using HRP-conjugated secondary antibodies. Antigen–antibody reaction was pictured with enhanced chemiluminescence (ECL; Sigma Aldrich, St Louis, MO, USA) kit under gel documentation system. image J software was used to evaluate the acquired images.
Normal and D-Limonene control groups showed irrelevant infarction area, whereas MI group revealed significant (p < 0.05) infarction area and higher heart to body ratio. On the other hand, animals pretreated with D-Limonene demonstrated infracted area deterring effects as well as decreased heart to body ratio. Expressive images of the TTC-stained heart slices and infarcted area extent were illuminated in Fig. 1.
Our results shown that cardiac homogenate level of CK-MB, CPK, cTnT and cTnI were significantly (p < 0.05) intensified in animals suffering from MI. However, with D-Limonene intervention, myocardial indicator enzymes dropped noticeably when related with the ISO induced MI group (p < 0.05), revealing that D-Limonene may improve myocardial damage resulted from MI as shown in Fig. 2.
Myocardial performance was identified via measuring blood pressure indices to indicate the cardiac tissue operational condition. Systolic Arterial Pressure, Diastolic Arterial Pressure and Mean Arterial Pressure were altered significantly in MI control group revealing the MI status (Fig. 3). Pretreatment with D-Limonene earlier to MI induction corrected markedly (p < 0.05) the myocardial performance as demonstrated by the improvment in blood pressure indices.
ERK signal transduction pathway displays an imperative part in myocardial injury [28]. Based upon this hypothesis, the influence of D-Limonene on the proteins involved in MAPK-ERK signal transduction including ERK, JNK and P38 were investigated via Western blotting (Fig. 4). The active Phosphorylated ERK, JNK was considerably augmented in MI animals compared to control groups suggesting that MI is associated with activation of MAPK-ERK signal transduction pathway (Fig. 4). While Limonene pretreatment, caused a decline in the protein expression of MAPK proteins pathaway. In addition, p-ERK/ERK ratio in the MI animals was significantly greater than in MI control groups, although this ratio dropped in D-Limonene treatment group.
Plentiful inflammatory cytokines including TNF-α, IL-6, NF-κB, and IL-1β were extensively augmented in animals suffering from MI, while management with D-Limonene obviously lessened (p < 0.05) the inflammatory cytokines levels (Fig. 5).
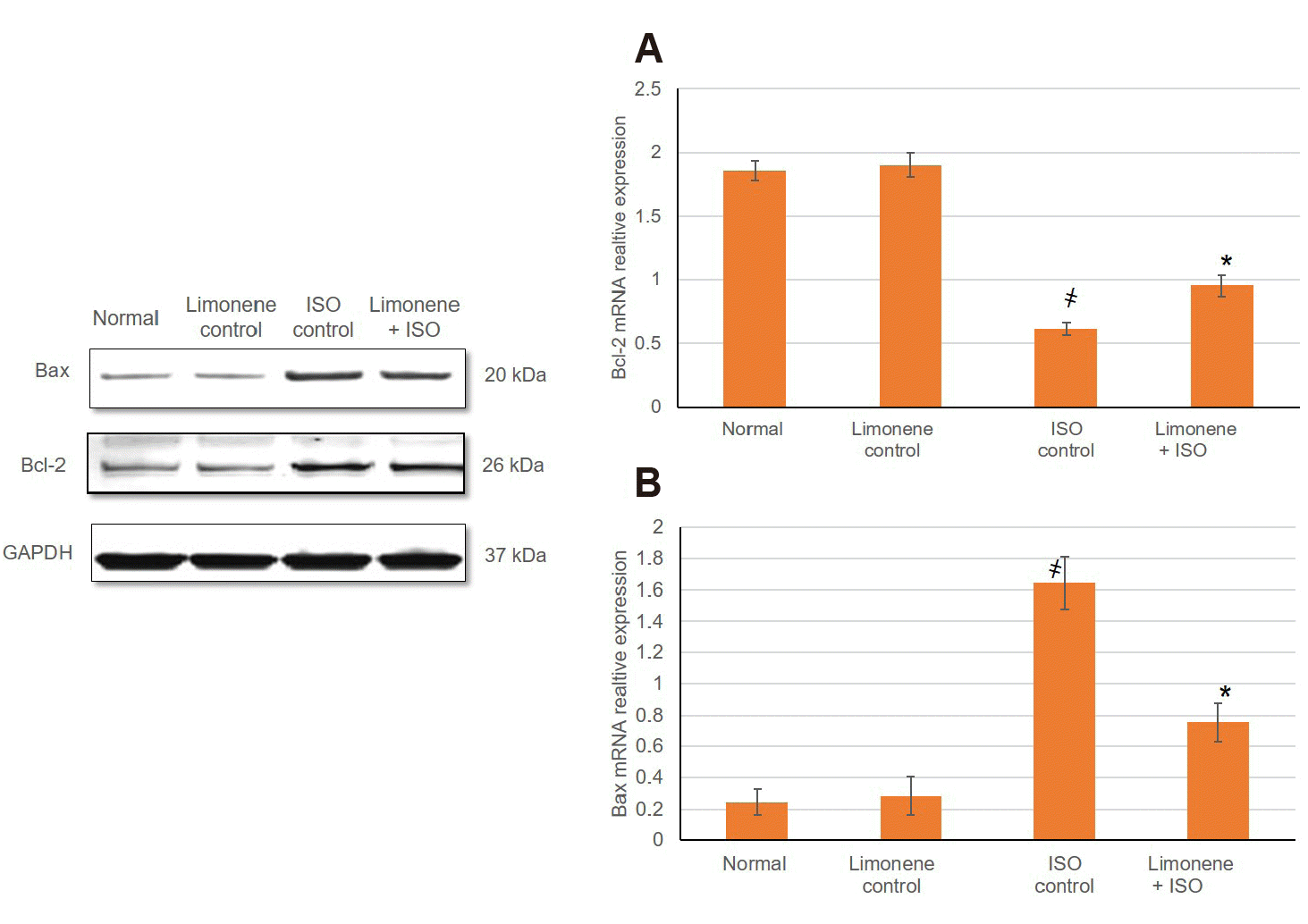
mRNA relative expression of anti-apoptotic protein (Bcl-2) and proapoptotic protein (Bax) were identified via RT-PCR as illustrated in Figure 6. The outcomes of our study disclosed that Bax mRNA expression level was significantly amplified, while Bcl-2 mRNA expression was remarkably inferior in MI animals (p < 0.05), demonstrating an apoptotic status arising with myocardium of MI group. In the animals pretreatment with D-Limonene, Bax relative mRNA expression deteriorated prominently, while Bcl-2 intensified, promoting that D-Limonene may constrain MI induced myocardial apoptosis.
In the current study, ISO-induced myocardial injury model were implemented to identify the cardio-protection actions of D-Limonene against myocardial infraction and to propose a mechanism of action for such protection. Significant infarction area, myocardial enzymes intensifications together with blood pressure alterations occurred in the experimental animals indicate clearly that ISO induced MI model was effectively generated in rats.
For more in depth exploration, earlier studies presented that numerous signaling pathways such as MAPK pathway proteins are tangled in ISO‐induced myocardial injury [28]. MAPK regulates the expressions of various apoptosis-related genes, such as Bcl-2 and Bax, which are key genes determining whether the apoptotic signaling pathway is activated after receiving extracellular stimulus signals in cells. So hereby in the present study, we tried to explore the effect of D-Limonene on myocardial hypertrophy and heart injury induced via ISO.
In the current study, ISO prompt apoptosis through an upregulation of JNK, p38 MAPK along with amplified pro-apoptotic proteins (Bax) and diminished ERK and anti-apoptotic protein (Bcl-2). In addition, we demonstrated an activation in ERK and JNK phosphorylation induced in MI animals. JNK and p38 MAPK subfamilies induced apoptosis and both were upregulated in MI animals causing inflammatory as well as apoptotic states within myocardial cells. While ERK which promotes cell survival was diminished within myocardium. Inhibiting MAPK pathway had revealed to protect against myocardial injury in the experimental animals as mentioned previously [21,22,29]. Triggering JNK was presented to have a pro‐apoptotic role in ISO induced apoptosis [30]. Also, reports have demonstrated that inhibiting ERK phosphorylation enhances reoxygenation and inhibits apoptosis and inflammatory response in myocardial cells [22].
Because of its pleasurable citrus fragrance, D-Limonene is extensively used as a flavor and essence additive in multiple fragrances, soaps and foods [1]. In the existing study, D-Limonene causes significant infarction area limitation, myocardial enzymes reduction together with improvement in blood pressure alterations in animals experienced MI indicating the cardio protection effects of D-Limonene on ISO induced MI model. Accordingly, we tried to understand the mechanisms lies behind this cardio protection.
Firstly, this study revealed that D-Limonene lessen numerous inflammatory mediators. Several reports documented Limonene anti-inflammatory actions through decreasing multiple inflammatory mediators levels such as TNF-a, IL-6, NF-κB, and IL-1β [2-5]. For instance, Kummer et al. [3] identified that Limonene reduced leukocytes infiltration and TNF-α in zymosan induced peritonitis in BALB/C mice. Also Limonene inhibited NO production, MMP-1 and MMP-13 in IL-1β activated human chondrocytes [5]. In addition, Limonene showed renal protective effect against Dox-induced renal damage [31], gastroprotection [2], and enhanced epithelial resistance in colonic HT-29/B6 cell monolayers [6] via attenuating inflammatory states. Another study provided that Limonene reserved the production of TH1 and TH2 cytokines by activated T cells [15]. For more in depth investigation, numerous mechanisms were proposed explaining why D-Limonene produces these actions. For instance, Tang et al. [32], proved that D-Limonene increased AMPKα 1/2 activation causing a decrease in NF-κB nuclear translocation, prevention of the target genes transcription including proinflammatory cytokines which defends PC12 cells against corticosterone-induced damage [32]. Another report proved that D-Limonene reduced prostaglandin E2 (PGE2) production, transforming growth factorβ (TGFβ) gene expression in ulcerative colitis rats [7].
Secondly, we tried to study D-Limonene anti-apoptotic effects in MI. In the current study, D-Limonene deteriorated Bax, while intensified Bcl-2, signifying that Limonene may constrain MI induced myocardial apoptosis status happening within the myocardium. Several studies supported the anti-apoptotic effect of D-Limonene [10,32]. For example, Bai et al. [10] demonstrated that D-Limonene has the ability to lessen the Bax/Bcl-2 ratio and so successfully, shields lens epithelial cells from Hydrogen Peroxide (H2O2)-induced apoptosis through inhibiting p38 MAPK phosphorylation.
Thirdly, we tried to study MAPK/NF-κB pathway as a possible pathway for explaining the cardio-protective effects of D-Limonene. Our data showed that D-Limonene markedly inhibited the ISO‐induced JNK/ERK signaling pathways, suggesting that the inhibitory effects of Limonene on JNK, ERK may contributed to cardio-protection. In addition, p-ERK/ERK ratio decreased in D-Limonene pretreated group suggesting that D-Limonene can effectively inhibit the excessive phosphorylation of ERK. Previous reports identified this pathway as a possible pathway though which D-Limonene produce several pharmacological actions. For example, Limonene attenuated the activation of NF-κB, ERK, JNK, and p38 MAPK signaling pathways, and prevented LPS-induced acute lung injury [33]. Another MAPK downstream transcription factor is NF-κB, which undergoes phosphorylated with subsequent increase in nuclear translocation, promoting transcription of numerous pro-inflammatory genes which provoke the myocardial damage and dysfunction. Current study demonstrated that ISO administration resulted in increased expression of NF-κB in myocardium, which further activates the release of extra inflammatory cytokines (TNF-α, IL-1β, and IL-6), and all these alterations were efficiently reversed via D-Limonene.
This is the first article revealing the molecular mechanisms via which D-Limonene enhances myocardial infraction injury to our knowledge. We identified that MAPK/NF-κB pathway inhibition with subsequent transcriptional regulation as well as D-Limonene anti-apoptotic effect together play a substantial role in mediating D-Limonene’s cardio-protective pharmacological actions. Additional studies are essential to establish the clinical application of D-Limonene in ischemic heart diseases patients.
ACKNOWLEDGEMENTS
The author also would like to express deepest gratitude for Dr. Maged ES Mohamed for reviewing the manuscript, supplying D-Limonene and for infinite support and encouragement.
Notes
REFERENCES
1. Sun J. 2007; D-Limonene: safety and clinical applications. Altern Med Rev. 12:259–264. PMID: 18072821.
2. de Souza MC, Vieira AJ, Beserra FP, Pellizzon CH, Nóbrega RH, Rozza AL. 2019; Gastroprotective effect of limonene in rats: influence on oxidative stress, inflammation and gene expression. Phytomedicine. 53:37–42. DOI: 10.1016/j.phymed.2018.09.027. PMID: 30668410.

3. Kummer R, Fachini-Queiroz FC, Estevão-Silva CF, Grespan R, Silva EL, Bersani-Amado CA, Cuman RK. 2013; Evaluation of anti-inflammatory activity of citrus latifolia tanaka essential oil and limonene in experimental mouse models. Evid Based Complement Alternat Med. 2013:859083. DOI: 10.1155/2013/859083. PMID: 23762165. PMCID: PMC3671226.
4. Hirota R, Roger NN, Nakamura H, Song HS, Sawamura M, Suganuma N. 2010; Anti-inflammatory effects of limonene from yuzu (Citrus junos Tanaka) essential oil on eosinophils. J Food Sci. 75:H87–92. DOI: 10.1111/j.1750-3841.2010.01541.x. PMID: 20492298.
5. Rufino AT, Ribeiro M, Sousa C, Judas F, Salgueiro L, Cavaleiro C, Mendes AF. 2015; Evaluation of the anti-inflammatory, anti-catabolic and pro-anabolic effects of E-caryophyllene, myrcene and limonene in a cell model of osteoarthritis. Eur J Pharmacol. 750:141–150. DOI: 10.1016/j.ejphar.2015.01.018. PMID: 25622554.

6. d'Alessio PA, Ostan R, Bisson JF, Schulzke JD, Ursini MV, Béné MC. 2013; Oral administration of D-limonene controls inflammation in rat colitis and displays anti-inflammatory properties as diet supplementation in humans. Life Sci. 92:1151–1156. DOI: 10.1016/j.lfs.2013.04.013. PMID: 23665426.
7. Yu L, Yan J, Sun Z. 2017; D-limonene exhibits anti-inflammatory and antioxidant properties in an ulcerative colitis rat model via regulation of iNOS, COX-2, PGE2 and ERK signaling pathways. Mol Med Rep. 15:2339–2346. DOI: 10.3892/mmr.2017.6241. PMID: 28260017.

8. Murali R, Karthikeyan A, Saravanan R. 2013; Protective effects of D-limonene on lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rats. Basic Clin Pharmacol Toxicol. 112:175–181. DOI: 10.1111/bcpt.12010. PMID: 22998493.
9. Roberto D, Micucci P, Sebastian T, Graciela F, Anesini C. 2010; Antioxidant activity of limonene on normal murine lymphocytes: relation to H2O2 modulation and cell proliferation. Basic Clin Pharmacol Toxicol. 106:38–44. DOI: 10.1111/j.1742-7843.2009.00467.x. PMID: 19796276.
10. Bai J, Zheng Y, Wang G, Liu P. 2016; Protective effect of D-limonene against oxidative stress-induced cell damage in human lens epithelial cells via the p38 pathway. Oxid Med Cell Longev. 2016:5962832. DOI: 10.1155/2016/5962832. PMID: 26682012. PMCID: PMC4670880.

11. Ahmad S, Beg ZH. 2013; Hypolipidemic and antioxidant activities of thymoquinone and limonene in atherogenic suspension fed rats. Food Chem. 138:1116–1124. DOI: 10.1016/j.foodchem.2012.11.109. PMID: 23411222.

12. Piccinelli AC, Morato PN, Dos Santos Barbosa M, Croda J, Sampson J, Kong X, Konkiewitz EC, Ziff EB, Amaya-Farfan J, Kassuya CA. 2017; Limonene reduces hyperalgesia induced by gp120 and cytokines by modulation of IL-1β and protein expression in spinal cord of mice. Life Sci. 174:28–34. DOI: 10.1016/j.lfs.2016.11.017. PMID: 27888114.
13. Panaskar SN, Joglekar MM, Taklikar SS, Haldavnekar VS, Arvindekar AU. 2013; Aegle marmelos Correa leaf extract prevents secondary complications in streptozotocin-induced diabetic rats and demonstration of limonene as a potent antiglycating agent. J Pharm Pharmacol. 65:884–894. DOI: 10.1111/jphp.12044. PMID: 23647682.
14. Ahmad SB, Rehman MU, Fatima B, Ahmad B, Hussain I, Ahmad SP, Farooq A, Muzamil S, Razzaq R, Rashid SM, Ahmad Bhat S, Mir MUR. 2018; Antifibrotic effects of D-limonene (5(1-methyl-4-[1-methylethenyl]) cyclohexane) in CCl4 induced liver toxicity in Wistar rats. Environ Toxicol. 33:361–369. DOI: 10.1002/tox.22523. PMID: 29251412.
15. Lappas CM, Lappas NT. 2012; D-Limonene modulates T lymphocyte activity and viability. Cell Immunol. 279:30–41. DOI: 10.1016/j.cellimm.2012.09.002. PMID: 23059811.

16. Yoon M. 2009; The role of PPARalpha in lipid metabolism and obesity: focusing on the effects of estrogen on PPARalpha actions. Pharmacol Res. 60:151–159. DOI: 10.1016/j.phrs.2009.02.004. PMID: 19646654.
17. Victor Antony Santiago J, Jayachitra J, Shenbagam M, Nalini N. 2012; Dietary d-limonene alleviates insulin resistance and oxidative stress-induced liver injury in high-fat diet and L-NAME-treated rats. Eur J Nutr. 51:57–68. DOI: 10.1007/s00394-011-0182-7. PMID: 21445622.

18. Moraes TM, Rozza AL, Kushima H, Pellizzon CH, Rocha LR, Hiruma-Lima CA. 2013; Healing actions of essential oils from Citrus aurantium and d-limonene in the astric mucosa: the roles of VEGF, PCNA, and COX-2 in cell proliferation. J Med Food. 16:1162–1167. DOI: 10.1089/jmf.2012.0259. PMID: 24328705.
19. Hassan MQ, Akhtar MS, Akhtar M, Ali J, Haque SE, Najmi AK. 2016; Edaravone, a potent free radical scavenger and a calcium channel blocker attenuate isoproterenol induced myocardial infarction by suppressing oxidative stress, apoptotic signaling and ultrastructural damage. Ther Adv Cardiovasc Dis. 10:214–223. DOI: 10.1177/1753944716630653. PMID: 26868288. PMCID: PMC5942626.

20. Lu L, Liu M, Sun R, Zheng Y, Zhang P. 2015; Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 72:865–867. DOI: 10.1007/s12013-015-0553-4. PMID: 25638347.

21. Verma VK, Malik S, Narayanan SP, Mutneja E, Sahu AK, Bhatia J, Arya DS. 2019; Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Mol Biol Rep. 46:1139–1148. DOI: 10.1007/s11033-018-04575-9. PMID: 30666500.

22. Ren GD, -Y Cui Y, Li WL, Li FF, Han XY. 2019; Research on cardioprotective effect of irbesartan in rats with myocardial ischemia-reperfusion injury through MAPK-ERK signaling pathway. Eur Rev Med Pharmacol Sci. 23:5487–5494. DOI: 10.26355/eurrev_201906_18218. PMID: 31298402.
23. Bi F, Xu Y, Sun Q. 2018; Catalpol pretreatment attenuates cardiac dysfunction following myocardial infarction in rats. Anatol J Cardiol. 19:296–302. DOI: 10.14744/AnatolJCardiol.2018.33230. PMID: 29724983. PMCID: PMC6280265.
24. Li L, Zhang LK, Pang YZ, Pan CS, Qi YF, Chen L, Wang X, Tang CS, Zhang J. 2006; Cardioprotective effects of ghrelin and des-octanoyl ghrelin on myocardial injury induced by isoproterenol in rats. Acta Pharmacol Sin. 27:527–535. DOI: 10.1111/j.1745-7254.2006.00319.x. PMID: 16626506.
25. Younis NS, Mohamed ME. 2019; β-caryophyllene as a potential protective agent against myocardial injury: the role of toll-like receptors. Molecules. 24:E1929. DOI: 10.3390/molecules24101929. PMID: 31109132. PMCID: PMC6572120.

26. Liu K, Li M, Ren X, You QS, Wang F, Wang S, Ma CH, Li WN, Ye Q. 2019; Huang Qi Tong Bi Decoction attenuates myocardial ischemia-reperfusion injury via HMGB1/TLR/NF-κB pathway. Mediators Inflamm. 2019:8387636. DOI: 10.1155/2019/8387636. PMID: 30944548. PMCID: PMC6421754.
27. Shahzad S, Mateen S, Naeem SS, Akhtar K, Rizvi W, Moin S. 2019; Syringic acid protects from isoproterenol induced cardiotoxicity in rats. Eur J Pharmacol. 849:135–145. DOI: 10.1016/j.ejphar.2019.01.056. PMID: 30731086.

28. Wang N, Guan P, Zhang JP, Li YQ, Chang YZ, Shi ZH, Wang FY, Chu L. 2011; Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, suppresses isoproterenol-induced heart failure in rats via JNK and ERK1/2 pathways. J Cell Biochem. 112:1920–1929. DOI: 10.1002/jcb.23112. PMID: 21433064.

29. Liu K, Wang F, Wang S, Li WN, Ye Q. 2019; Mangiferin attenuates myocardial ischemia-reperfusion injury via MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid Med Cell Longev. 2019:7285434. DOI: 10.1155/2019/7285434. PMID: 31249649. PMCID: PMC6535818.

30. Krishnamurthy P, Subramanian V, Singh M, Singh K. 2007; Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertension. 49:865–872. DOI: 10.1161/01.HYP.0000258703.36986.13. PMID: 17283249.
31. Rehman MU, Tahir M, Khan AQ, Khan R, Oday-O-Hamiza , Lateef A, Hassan SK, Rashid S, Ali N, Zeeshan M, Sultana S. 2014; D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med (Maywood). 239:465–476. DOI: 10.1177/1535370213520112. PMID: 24586096.

32. Tang XP, Guo XH, Geng D, Weng LJ. 2019; d-Limonene protects PC12 cells against corticosterone-induced neurotoxicity by activating the AMPK pathway. Environ Toxicol Pharmacol. 70:103192. DOI: 10.1016/j.etap.2019.05.001. PMID: 31103492.

33. Chi G, Wei M, Xie X, Soromou LW, Liu F, Zhao S. 2013; Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation. 36:501–511. DOI: 10.1007/s10753-012-9571-1. PMID: 23180366.

Fig. 1
(A) Influence of D-Limonene pretreatment (50 mg/kg) on heart to body weight ratio, (B) TTC-stained heart illustrating the influence of D-Limonene pretreatment (50 mg/kg) for 21 days on infarcted area extent.
Values were indicated as mean ± standard deviation (n = 6). MI, myocardial infarction; ISO, isoproterenol; TTC, Triphenyl Tetrazolium Chloride. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.
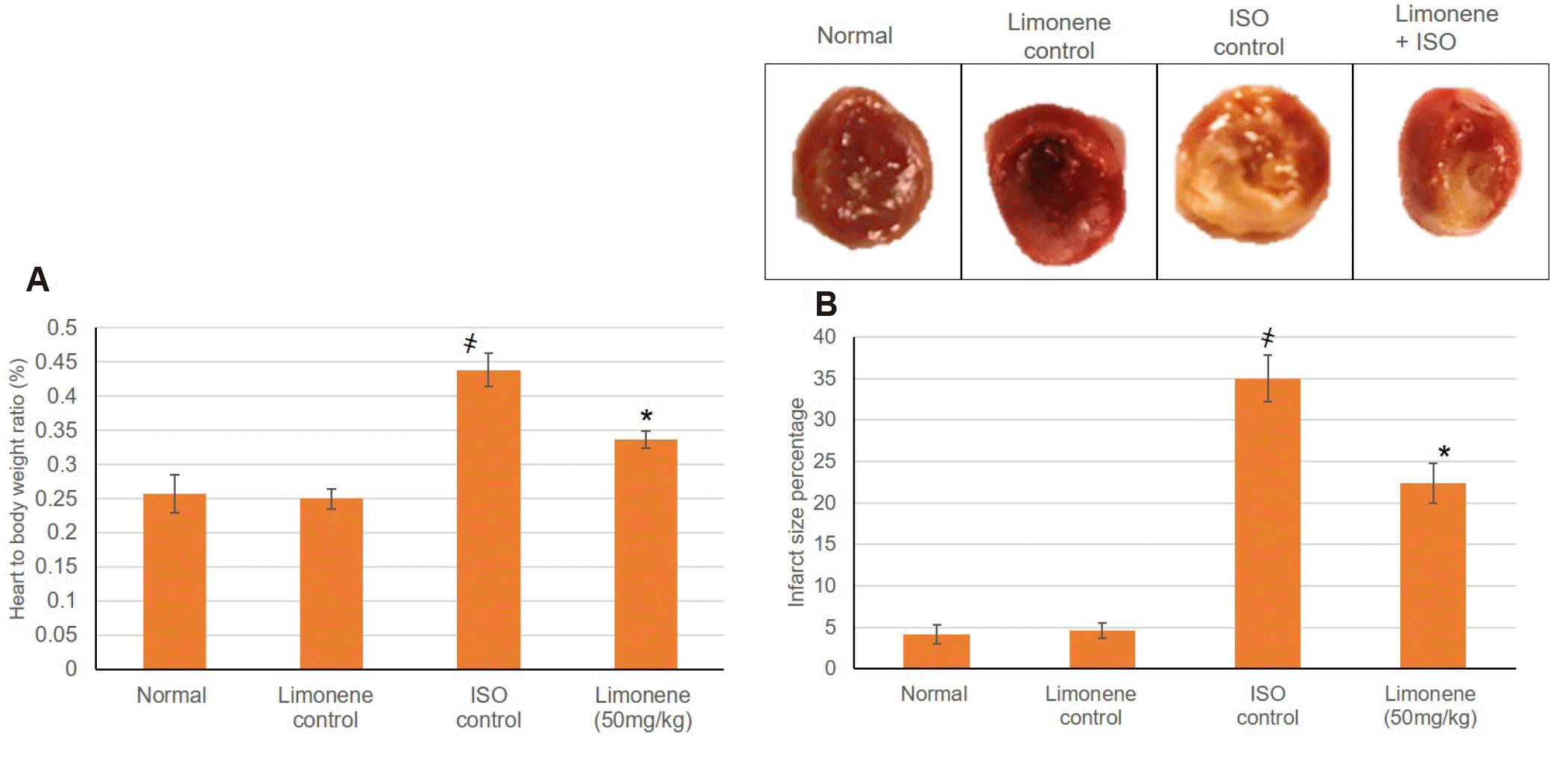
Fig. 2
Influence of D-Limonene pretreatment (50 mg/kg) for 21 days on cardiac injury markers in ISO induced MI:
(A) CPK, (B) CK-MB, (C) cTnI and (D) cTnT. Values were indicated as mean ± standard deviation (n = 6). MI, myocardial infarction; ISO, isoproterenol; CPK, Creatine Phosphokinase; CK-MB, Creatine Kinase-Myocardial Bound; cTnI, Cardiac Tropinine I; cTnT, Cardiac Troponin T. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.
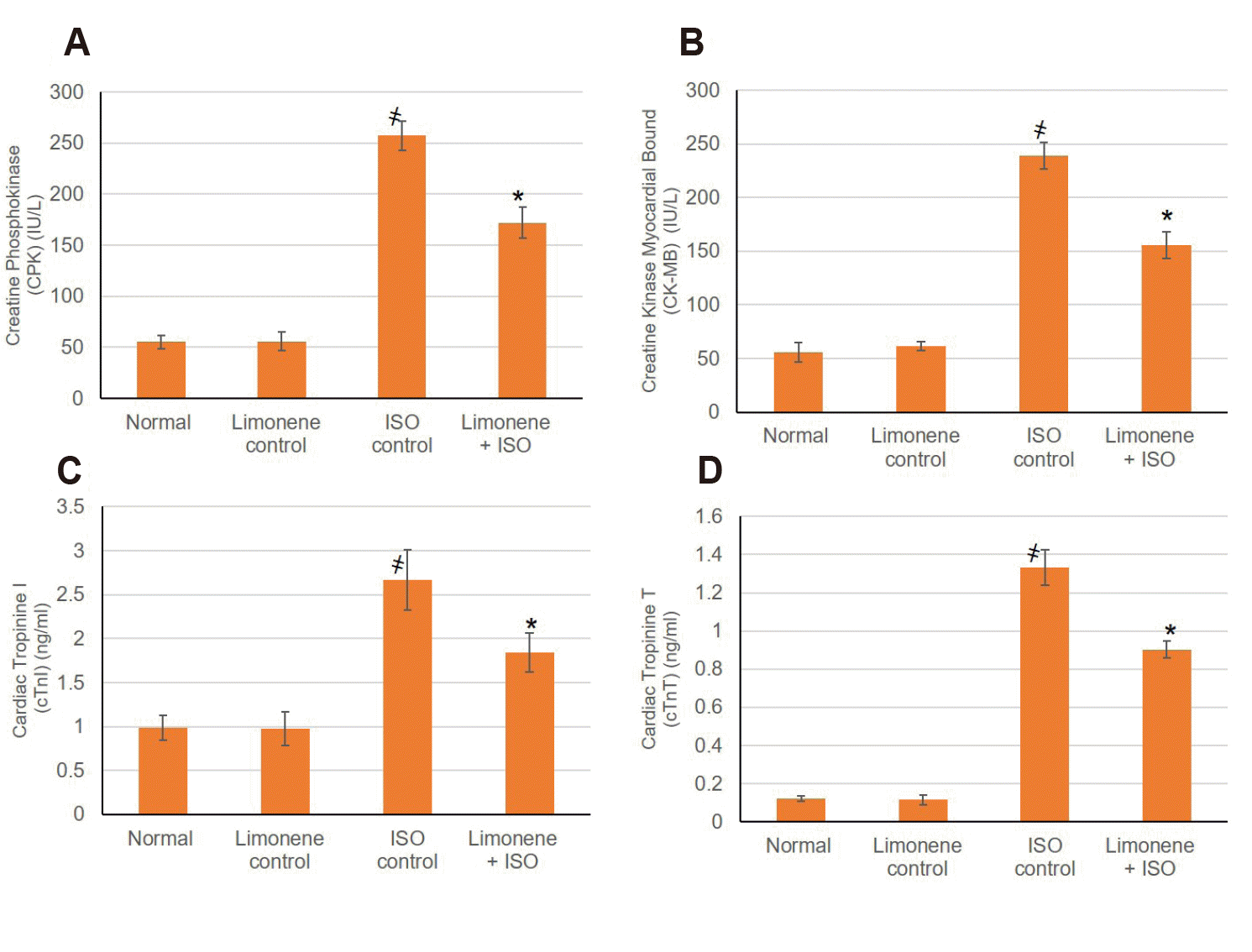
Fig. 3
Influence of D-Limonene pretreatment (50 mg/kg) for 21 days on blood pressure indices.
(A) SAP, (B) DAP, and (C) MAP. Values were indicated as mean ± standard deviation (n = 6). SAP, Systolic Arterial Pressure; DAP, Diastolic Arterial Pressure; MAP, Mean Arterial Pressure; MI, myocardial infarction; ISO: isoproterenol. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.
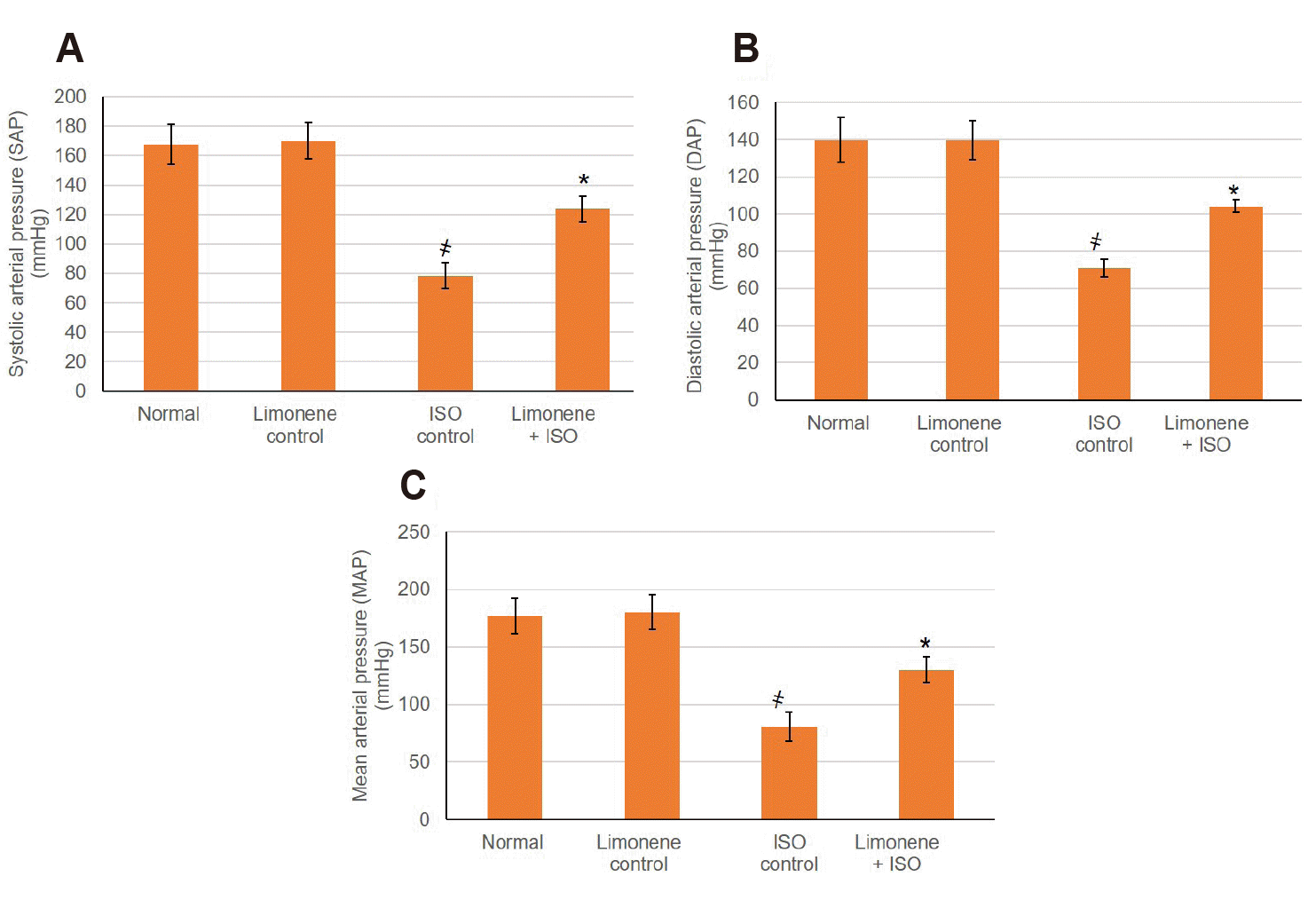
Fig. 4
Influence of D-Limonene pretreatment (50 mg/kg) for 21 days on protein expression of (A) p-ERK/ERK (B) p-JNK/JNK and (C) p-p38/p38 ratios in ISO induced MI.
Values were indicated as mean ± standard deviation (n = 6). ERK, extracellular signal-regulated kinase; JNK, c-Jun-N-terminal kinase; ISO: isoproterenol; MI, myocardial infarction. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.
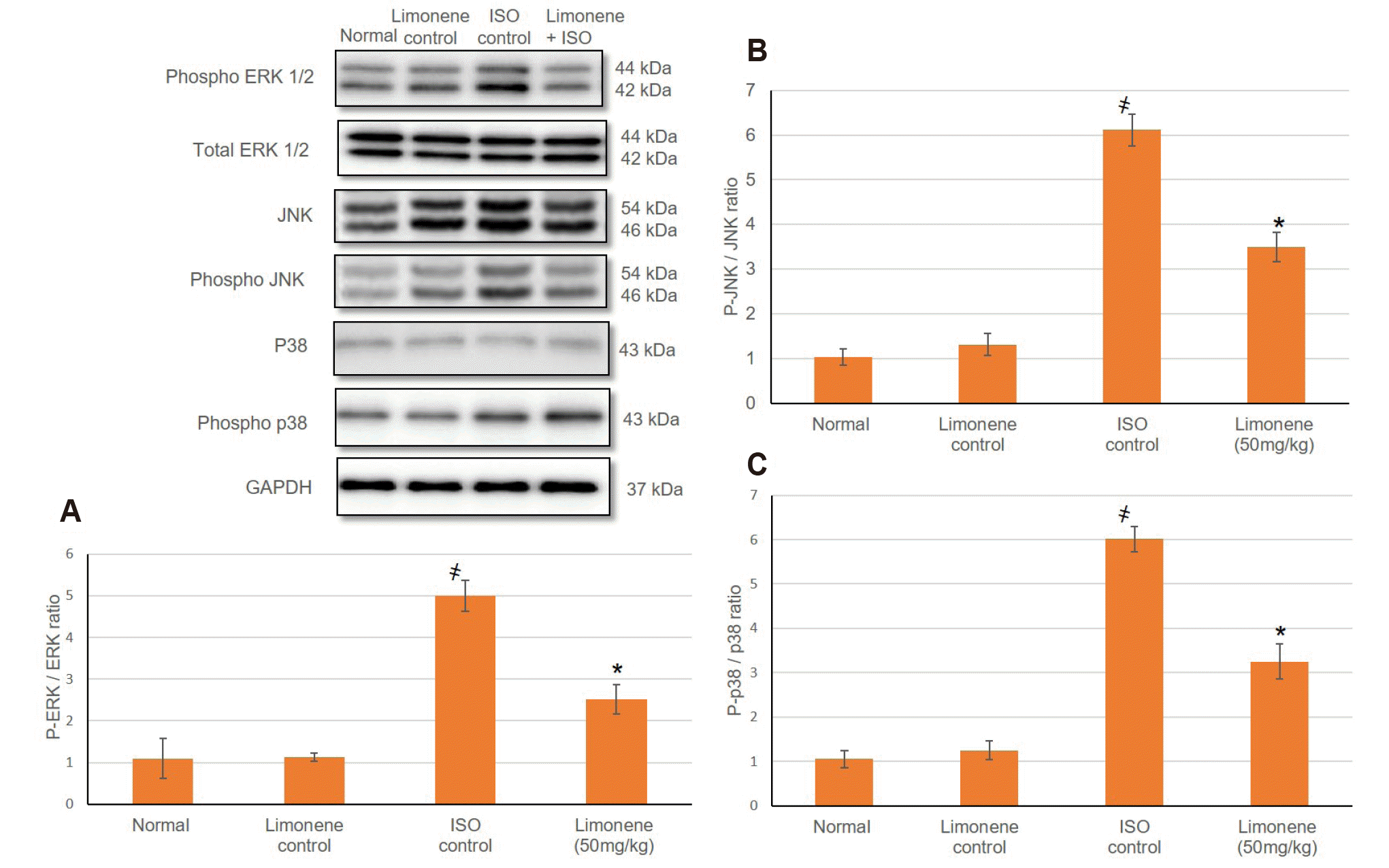
Fig. 5
Influence of D-Limonene pretreatment (50 mg/kg) for 21 days on cardiac inflammatory markers.
(A) IL-1β, (B) IL-6 (C) TNF-α, and (D) NF-κB. Values were indicated as mean ± standard deviation (n = 6). IL, interleukin; TNF, tumor necrosis factor; MI, myocardial infarction; ISO: isoproterenol. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.
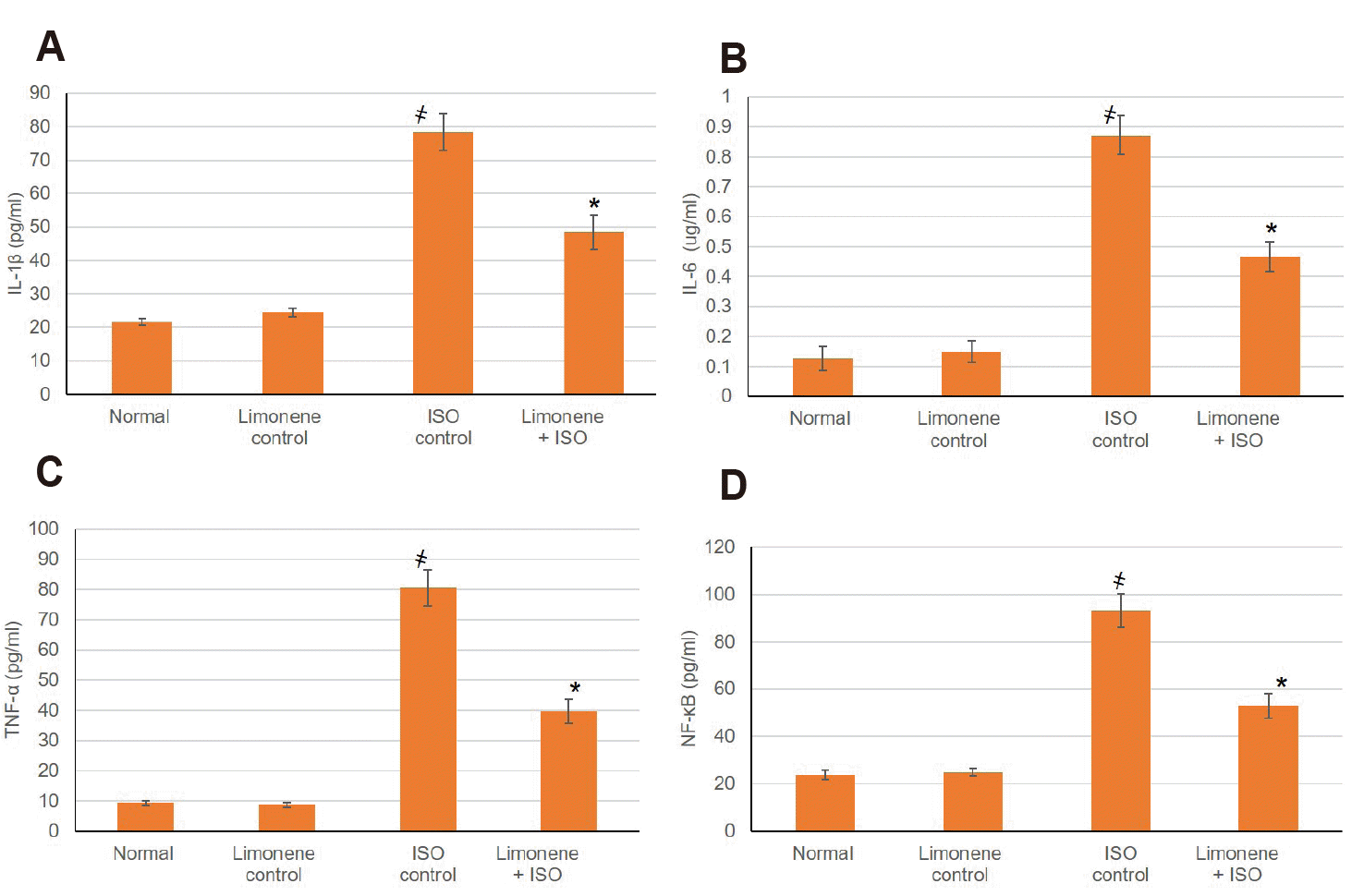
Fig. 6
(A) Influence of D-Limonene pretreatment (50 mg/kg) on Bcl-2 mRNA expression, (B) Influence of D-Limonene pretreatment (50 mg/kg) on Bax mRNA expression.
Values were indicated as mean ± standard deviation (n = 6). MI, myocardial infarction; ISO: isoproterenol. Probability values (p < 0.05): where ҂designates statistically significant compared to normal animals, *designates statistically significant compared to MI animals using one-way ANOVA followed by Tukey’s test as a post-hoc analysis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download