Abstract
Collision tumors are extremely rare, and currently, no therapeutic protocols are established. A 64-year-old man presented to his physician with complaints of right chest and abdominal pain. The contrast-enhanced CT scan showed a mass measuring 3.6 cm around the gastric fundus. Esophagogastroduodenoscopy was performed and a semicircular longitudinal ulcerative mass was found at the distal esophagus. A mass measuring about 4 cm with central ulceration was noted at the cardia. The esophageal biopsy revealed positivity for a component of neuroendocrine carcinoma adjacent to a squamous cell carcinoma. PET-CT revealed a mass in the esophagus and cardia and several tumors in the whole liver, pancreas, and bone. The patient was finally diagnosed with a collision tumor of the esophagus with multiple metastases. In conclusion, patients with collision tumors must undergo active multidisciplinary management that will include pathologists and oncologists, who will decide on proper treatment strategies.
Esophageal cancer is the seventh most common cancer and is the sixth-leading cause of cancer mortality worldwide.1 Surgical treatment techniques and chemotherapy agents have been developed to treat these tumors, but the overall mortality rate of esophageal cancer remains higher than that of other cancers.2 The two main types of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma.3 Other types of esophageal cancer are rare and include lymphomas, sarcomas, melanomas, and neuroendocrine tumors.4
Mixed tumors can be classified as composite and collision neoplasms. Collision tumors are defined as those that arise from incidental meeting and without significant intermingling of two independent neoplasms.5 These tumors usually confer a poor prognosis since both components are malignant. There are very few case reports and case series concerning collision tumors located in the esophagus. Herein, we describe a patient with a collision tumor of the distal esophagus.
A 64-year-old man presented to Ewha Womans University Seoul Hospital with complaints of right chest, abdominal pain and dysphagia. He had experienced multiple traumatic rib fractures 6 months prior and he received conservative management at a local hospital. Recently, the pain had not subsided even when administering opioid painkillers, and he was transferred to Ewha Womans University Seoul Hospital for further evaluation. He complained of progressive dysphagia to solid foods rather than liqids. He denied having undergone previous surgeries or any past history or family history of cancer. He had an initial blood pressure of 139/97 mmHg, a pulse rate of 143 beats/min, a body temperature of 36.6℃, and a respiratory rate of 22 breaths/min. The physical examination revealed direct tenderness at the right ribs. Non-specific findings were noted on the abdominal physical examination. The laboratory test results were as follows: a white blood cell count of 9,580 cells/mm3, a hemoglobin level of 12.6 g/dL, and a platelet count of 193×103 cells/mm3. No abnormality of his electrolytes was noted. Elevated levels of AST (98 U/L), ALT (121 U/L), ALP (457 U/L), and LDH (2,636 U/L) were showed. The serum levels of tumor markers such as CEA (47 ng/mL) and CA 19-9 (65 U/mL) were increased, whereas the AFP level (3.5 ng/mL) was within the normal range. His chest X-ray showed multiple acute fractures of the right ribs. Chest CT revealed no abnormalities. A contrast-enhanced CT scan of the abdomen and pelvis showed a mass measuring 3.6 cm around the gastric fundus portion and multiple liver and lymphatic metastases in the portal hepatis and periportal areas. Dissemimated bone metastasis at whole spines, sternum, ribs, and both pelvic bones were noted on the abdomen and pelvis CT (Fig. 1). Esophagogastroduodenoscopy was performed and a semicircular longitudinal ulcerative mass was found to be located at the distal esophagus (34-37 cm from the upper incisor). A mass measuring about 4 cm in diameter with central ulceration was noted at the cardia (Fig. 2). An intensely hypermetabolic esophageal mass involving the gastroesophageal junction (standardized uptake value [SUV] 11.0) was noted on PET-CT. In addition, multiple hypermetabolic lesions were detected in the whole liver (SUV 14.5), bones, and lymph nodes (SUV 11.5) at the left gastric, portocaval, and porta hepatis areas (Fig. 3). Histopathological examination of an endoscopic esophageal biopsy specimen showed small cell neuroendocrine carcinoma (NEC) and SCC in a colliding pattern. The gastric mass in the cardia was grossly suspected to be a malignant gastrointestinal stromal tumor, but the finding was inconclusive. Immunohistochemistry showed p63 (+) and p16 (+) in the squamous carcinoma cells and cluster of differentiation-56 (+), synaptophysin (+), and thyroid transcription factor-1 (+) in the NEC cells (Fig. 4). Therefore, the final diagnosis was a collision tumor of the distal esophagus (T3N3M1, clinical stage IV). He was transferred to the oncology department for palliative chemotherapy. The patient was treated with palliative chemotherapy using a combination of etoposide (100 mg/m2 for 3 days) and cisplatin (60 mg/m2 for 3 days). He died 2 months later due to his suppressed immune condition after the first course of chemotherapy.
Collision tumors are rare clinical entities wherein two different types of tumors occur at the same anatomic site. Each of the tumors has sharply different behavioral and histological characteristics. In contrast, composite tumors are comprised of two tumors of different origins, pathologies, and phenotypes. These two types of tumors are in close proximity with actual histologic merging of the different types of tumor cells.6 In our case, histological examination showed squamous and neuroendocrine components of the tumor with distinctive borders. Thus, we diagnosed the patient as having a collision tumor.
The pathogenesis of collision tumor has not been extensively investigated and remains unclear. The hypotheses relating to the development of collision tumors are as follows: 1) a concurrently proliferation of two different cell lines; 2) a common precursor stem cell that differentiates into other cell typesthat keep their own individual properties; and 3) malignant deformations in the local microenvironment of an original tumor accelerate the development of a second distinct tumor adjacent to it.7-9 Collision tumors are thought to be difficult to diagnose prior to resection and they are most often diagnosed on the basis of histopathological and immunohistochemical examination.10
According to a systemic review,11 esophageal collision tumors have been presented in 17 cases over the past two decades (Table 1). Most of these cases involved men, and the mean age of the patients was in the early sixties. These tumors were usually located at the distal esophagus. Mixed NEC and SCC tumors like that in our case were only noted in two cases. Regional lymph nodes were infiltrated in 47.1% of the cases, and all of the patients underwent surgical resection. Twelve patients received chemotherapy as neo- or adjuvant therapy. Four patients died during follow-up, with a median survival time of 12.5 months.
The esophageal tumor in our case consisted of two distinct lesions; small cell type NEC on the left side and well differentiated SCC on the right side slide. Our case involved small cell type NEC, which accounts for 0.6-2.8% of all esophageal tumors.12 Its prevalence is the highest in southeast Asian countries, and it is highly aggressive, rapidly progressive, and confers a poor prognosis.13 Curative resection is not a treatment option for most high grade metastatic NECs of the gastrointestinal system.14 The National Comprehensive Cancer Network recommends administering either carboplatin+etoposide or cisplatin+irinotecan, which are derived from regimens that are normally used for treating small cell lung carcinoma, and this is likely due to their clinical and histopathological similarity.15 For collision tumors, the chemotherapy regimen is determined based on NEC with poor prognosis. Collision tumors require more complex management, including administration of a patient-tailored chemotherapy according to the histology of the tumor. We recommend that patients with collision tumors undergo aggressive multidisciplinary oncologic management that may include systemic therapy and well-selected surgical management.
In conclusion, collisions tumors of the esophagus are rare malignancies with an uncertain pathophysiology and treatment strategies. Due to their non-specific symptoms and metastatic potential, it is important for physicians to remain cognizant of collision tumor when making their differential diagnosis. Patients with collision tumors must undergo active multidisciplinary management with pathologists and oncologists, and this management may include administration of a personalized chemotherapy regimen that is based on the histologic findings of the particular tumor.
REFERENCES
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. 2018; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. DOI: 10.3322/caac.21492. PMID: 30207593.

2. Gupta B, Kumar N. 2017; Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur J Cancer Prev. 26:107–118. DOI: 10.1097/CEJ.0000000000000249. PMID: 27014938.

3. Short MW, Burgers KG, Fry VT. 2017; Esophageal cancer. Am Fam Physician. 95:22–28. PMID: 28075104.
4. Zielinski J, Kruszewski WJ, Jaworski R, et al. 2012; Rare oesophageal tumours: experience of one centre. Eur Surg. 44:361–365. DOI: 10.1007/s10353-012-0165-9. PMID: 23440953. PMCID: PMC3573716.

5. Yao B, Guan S, Huang X, Su P, Song Q, Cheng Y. 2015; A collision tumor of esophagus. Int J Clin Exp Pathol. 8:15143–15146. PMID: 26823858. PMCID: PMC4713644.
6. Michalinos A, Constantinidou A, Kontos M. 2015; Gastric collision tumors: an insight into their origin and clinical significance. Gastroenterol Res Pract. 2015:314158. DOI: 10.1155/2015/314158. PMID: 25767509. PMCID: PMC4342179.

7. Sung CT, Shetty A, Menias CO, et al. 2017; Collision and composite tumors; radiologic and pathologic correlation. Abdom Radiol (NY). 42:2909–2926. DOI: 10.1007/s00261-017-1200-x. PMID: 28623377.

8. Brahmania M, Kanthan CS, Kanthan R. 2007; Collision tumor of the colon-- colonic adenocarcinoma and ovarian granulosa cell tumor. World J Surg Oncol. 5:118. DOI: 10.1186/1477-7819-5-118. PMID: 17949502. PMCID: PMC2164962.

9. Milne AN, Carvalho R, van Rees BP, van Lanschot JJ, Offerhaus GJ, Weterman MA. 2004; Do collision tumors of the gastroesophageal junction exist? A molecular analysis. Am J Surg Pathol. 28:1492–1498. DOI: 10.1097/01.pas.0000138184.74496.4d. PMID: 15489653.
10. Ciobanu D, Meşină C, Streba L, et al. 2014; The role of immunohistochemistry in diagnosing a synchronous colon tumor. Rom J Morphol Embryol. 55(3 Suppl):1209–1213. PMID: 25607408.
11. Schizas D, Katsaros I, Michalinos A, et al. 2018; Collision tumors of the gastrointestinal tract: a systematic review of the literature. Anticancer Res. 38:6047–6057. DOI: 10.21873/anticanres.12955. PMID: 30396919.

12. Yang L, Sun X, Zou Y, Meng X. 2014; Small cell type neuroendocrine carcinoma colliding with squamous cell carcinoma at esophagus. Int J Clin Exp Pathol. 7:1792–1795. PMID: 24817981. PMCID: PMC4014265.
13. Casas F, Ferrer F, Farrús B, Casals J, Biete A. 1997; Primary small cell carcinoma of the esophagus: a review of the literature with emphasis on therapy and prognosis. Cancer. 80:1366–1372. DOI: 10.1002/(SICI)1097-0142(19971015)80:8<1366::AID-CNCR2>3.0.CO;2-D. PMID: 9338459.
14. Fujihara S, Kobayashi M, Nishi M, et al. 2018; Composite neuroendocrine carcinoma and squamous cell carcinoma with regional lymph node metastasis: a case report. J Med Case Rep. 12:227. DOI: 10.1186/s13256-018-1775-z. PMID: 30139375. PMCID: PMC6108124.

15. Kamei K, Shindoh J, Kiya Y, Matsumoto I, Hashimoto M, Takeyama Y. 2019; Oct. 15. Conversion surgery after extensive chemotherapy for stage IV mixed adenoneuroendocrine carcinoma (MANEC) of the gallbladder: clinical implications from the patterns of response and recurrence. Clin J Gastroenterol. [Epub ahead of print]. DOI: 10.1007/s12328-019-01053-y. PMID: 31617127.

FIGURES AND TABLE
Fig. 1
Abdominal computed tomography findings. (A) About a 3.6 cm mass around the gastric fundus portion and also liver metastasis. (B) Multiple bone metastasis in the vertebra and sacrum.
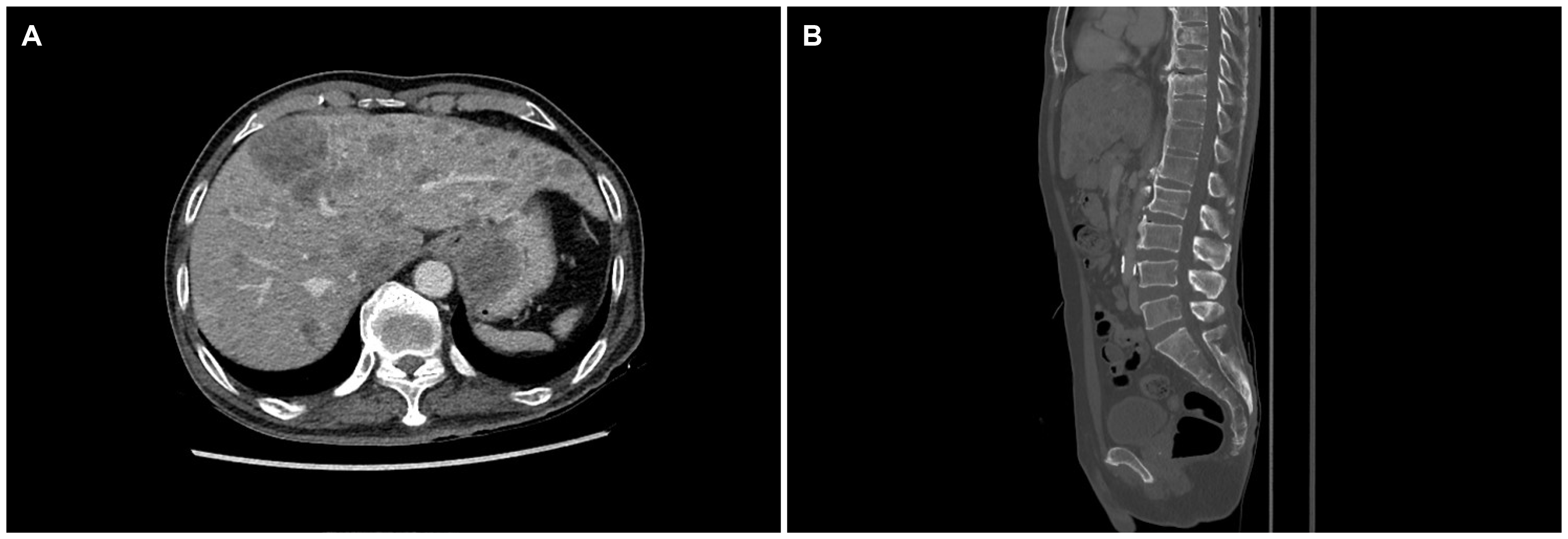
Fig. 2
Endoscopic images of the esophageal neoplasm. (A, B) A semicircular longitudinal ulcerative mass at the distal esophagus. (C) About a 4 cm sized subepithelial mass with central ulceration at the fundus.
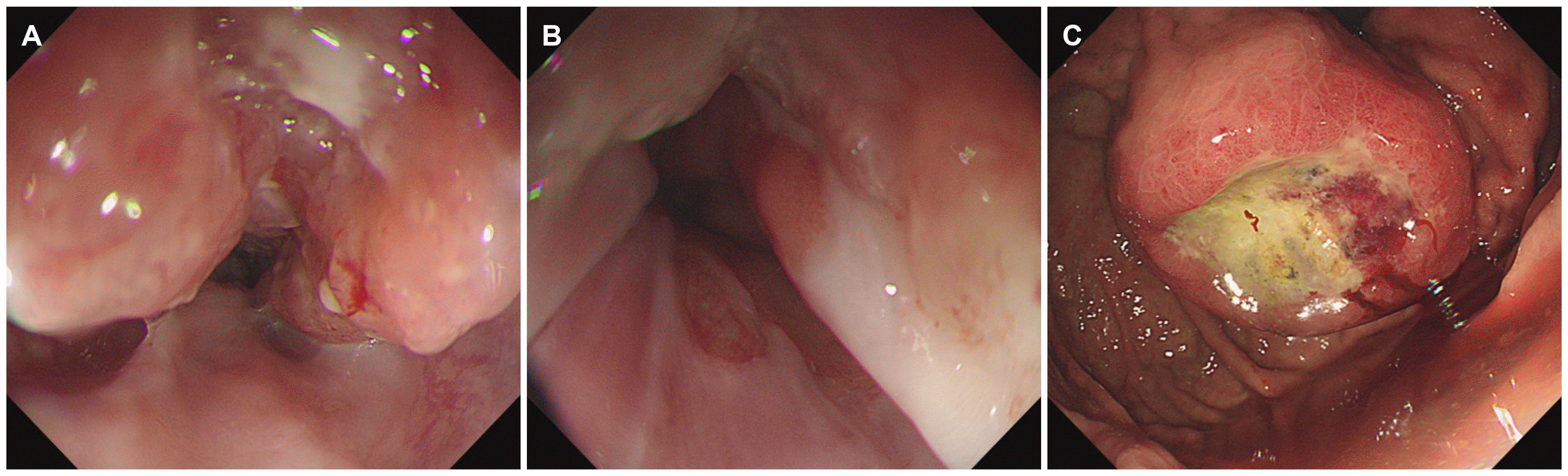
Fig. 3
Positron emission tomography-computed tomography findings. (A-C) A hypermetabolic lesion in the esophagus and cardia, and several tumors in the whole liver, pancreas, and bone.
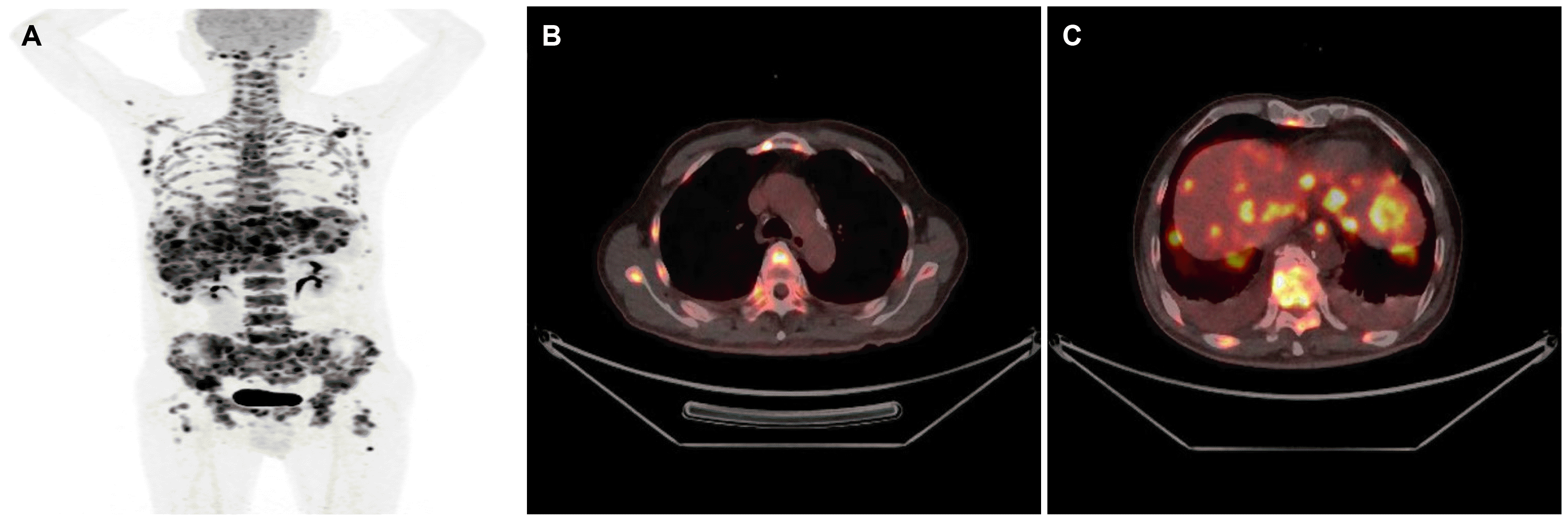
Fig. 4
Histologic features of the esophagus specimen. (A) The positive for the component of the neuroendocrine carcinoma adjacent to a squamous carcinoma without intermigling (H&E, ×400). (B) Strong staining of p63 (+) for the squamous carcinoma cells (Immunohistochemical staining, ×400). (C) Strong staining of CD56 (+) for the neuroendocrine carcinoma cells (Immunohistochemical staining, ×400).
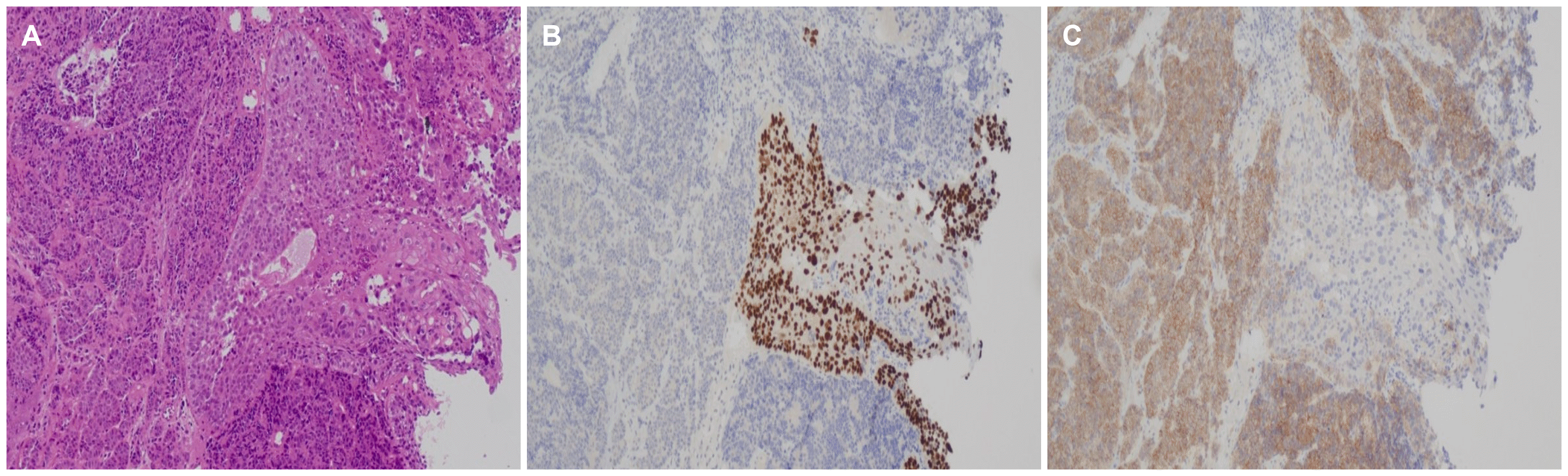
Table 1
Clinical Characteristics, Pathology, and Treatment Options of Esophageal Collision Tumors




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download