This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Carbapenemase-producing Enterobacteriaceae (CPE) are highly drug-resistant pathogens. Screening the contacts of newly-identified CPE patients is crucial for nosocomial transmission control. We evaluated the acquisition rate of CPE in close contacts as a function of CPE genotype.
Materials and Methods
This study was conducted in Asan Medical Center, a 2,700-bed, tertiary teaching hospital in Seoul, Korea, between November 2010 and October 2017. Index cases were defined as patients with positive tests for CPE from any infected or colonized site during hospitalization who had no direct epidemiologic linkage with existing CPE patients; close contact patients were defined as those whose hospital stay overlapped with the stay of an index case for at least one day and who occupied the same room or intensive care unit (ICU). Secondary patients were defined as those who produced positive CPE culture isolates from surveillance cultures that had the same CPE enzyme as that of the index case patients.
Results
A total of 211 index case patients and 2,689 corresponding contact patients were identified. Of the contact patients, 1,369 (50.9%) including 649 New-Delhi metallo-beta-lactamase-1 (NDM-1) and 448 Klebsiella pneumoniae carbapenemase (KPC)-producing CPE exposures were screened, and 44 secondary patients (3.2%; 95% confidence interval 2.3 - 4.3%) were positive for NDM-1-producing CPE (16 patients) and KPC-producing (24 patients) CPE. The CPE acquisition rate (5.4%) for KPC-producing CPE exposures was significantly higher than that for NDM-1 exposures (2.7%) (P = 0.01).
Conclusion
The CPE acquisition rate was 3.2% among close contacts sharing a multi-patient room, with about a two-fold higher risk of KPC-producing CPE than NDM-1-producing CPE.
Keywords: Carpapenemase-producing Enterobacteriaceae, Acquisition rate, Klebsiella pneumoniae carbaepenamse, New Delhi Metallo-beta-lactamase-1
Introduction
As the use of carbapenem has increased, the incidence of carbapenem-resistant
Enterobacteriaceae (CRE) infections has also increased. There are several mechanisms which confer carbapenem resistance on CRE, including structural mutations [
1] or production and release of carbapenemase [
2]. Particularly, the production of carbapenem-hydrolyzing β-lactamase enzyme (carbapenemase-producing
Enterobacteriaceae; CPE) is a concern because it is easy to spread to patients and it is difficult to treat infections [
3]. Therefore, CPE have been a great threat to the treatment of nosocomial infections [
4].
Since the first CPE outbreaks in our hospital in 2010, our institute has strict contact precautions and screening tests for in-hospital patients who have been in close contact with CPE-infected or -colonized patients to allow early detection of secondary transmission. Among various carbapenemase types, New Delhi Metallo-beta-lactamase-1 (NDM-1) and
Klebsiella pneumoniae carbapenemase (KPC)-producing strains have been the most dominant [
5]. In the present work, we evaluated how the rate of acquisition of carbapenem-resistance by close contact in terms of sharing a multi-patient room depends on the carbapenemase genotype, especially in the cases of NDM-1 and KPC strains.
Materials and Methods
Index patients with confirmed CPE infection and other patients in close contact patient with index patients were prospectively enrolled in this study at Asan Medical Center, a 2,700-bed tertiary hospital in Seoul, Korea, from January 2010 to December, 2017. The study protocol was approved by the institutional review board of Asan Medical Center (IRB number 2019-1571). Index case patients were defined as those with positive tests for CPE during their hospitalization without any direct epidemiologic linkage (
i.e. sharing of location, outbreak setting) with existing CPE patients, and close contact patients were defined as those whose hospital stay overlapped with that of an index case present in the same hospital room at least one day before confirmation with culture results. If an index patient was located in the intensive care unit (ICU), the definition of close contact patients was expanded to include other patients present in the same open space of the ICU that the index patient occupied. Secondary patients were defined as those who produced positive CPE culture isolates from surveillance cultures that had the same CPE enzyme as that of the index case patients. We did not investigate close contacts in the emergency room because it was difficult to identify close contact patients. Exposure time was defined as the time from the first day when the close contact patient and the index patient came into the same place to the time of isolation of index case. Patients with hospital-acquired infections were defined as those who had positive cultures results more than 48 hours after hospitalization. Patients with community-acquired infections were defined as those who had positive culture results within 48 hours of admission. If patients were transferred from another medical institution, infections were defined as healthcare-associated infections [
6]. Invasive infections were defined by reference to the Korean Nosocomial Infections Surveillance System [
7]. Colonization was defined as CPE confirmed in a rectal swab or stool culture in patients without any clinical symptoms of
Enterobacteriaceae infections.
For microbiological evaluation, we used MacConkey agar plates supplemented with imipenem (1 mg/L) and the Microscan (Omron Microscan systems Inc., Renton, WA, USA) system for surveillance cultures. We performed genotyping PCR after we performed phenotyping test of carbapenemase using the modified Hodge test (MHT), Ethylenediaminetetraacetic acid (EDTA) test, and boric acid test. Microbiologic assays took approximately 3-4 days to complete, and close contact patients were only screened for CPE once [
8].
If Enterobacteriaceae identified in culture showed intermediate or higher resistance two or more disks, carbapenem class antibiotics with imipenem or meropenem, we identified antimicrobial resistance by disk diffusion.
Our hospital does not implement active surveillance for CPE on admission except in outbreak situations.
SPSS version 21.0 for Windows (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. Categorical variables were analyzed by the Chi-square test or Fisher’s extract test, as appropriate. Continuous variables were analyzed by the independent samples t-test or the Mann-Whitney U-test. Logistic regression analysis was performed to evaluate the effect of independent variables on risk. Two-tailed P values <0.05 were considered statistically significant.
Results
1. Study subjects
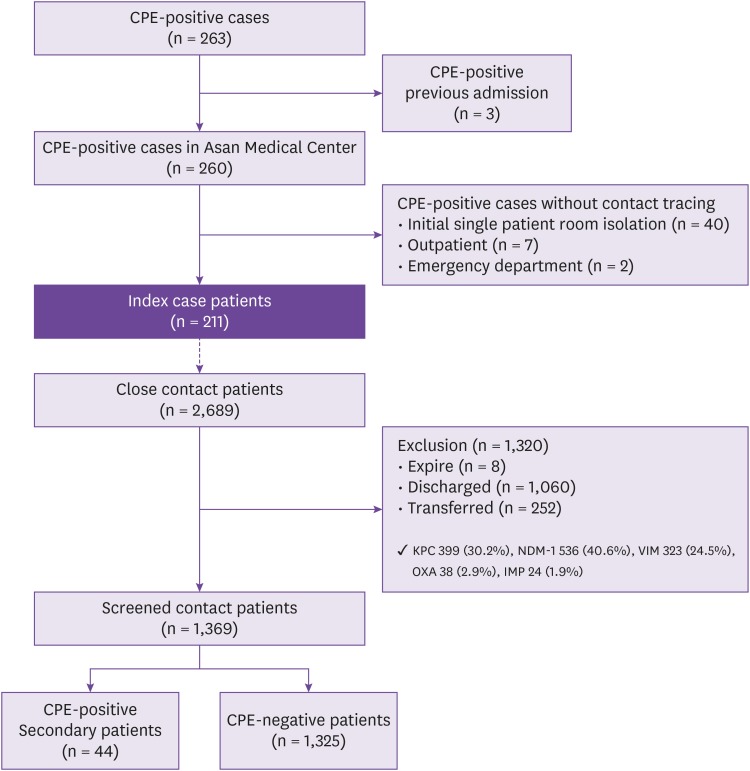
Among a total of 263 patients with confirmed CPE infection or colonization, we excluded three patients who were CPE-positive from previous admissions and 49 patients who did not undergo contact tracing for any reason (i.e., were placed in a single patient room, visited the outpatient clinic or emergency department without being admitted, etc.). Finally, a total of 211 index patients with positive CPE results from their clinical specimens were enrolled.
The 211 index case patients were found to have been in close contact with a total of 2,689 patients. Of these 2,689 contact patients, 1,320 (49%) were excluded because of discharge or death; the remaining 1,369 in-hospital patients (51%) underwent active CPE surveillance tests and 44 (3.2%) secondary CPE patients were identified (
Fig. 1).
Figure 1
Flow chart of the Study.
CPE, Carbapenemase producing Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase; NDM-1, New Delhi metallo- beta-lactamase; VIM, verona integron-encoded metallo-beta-lactamase; OXA, oxacillin carbapenemase.
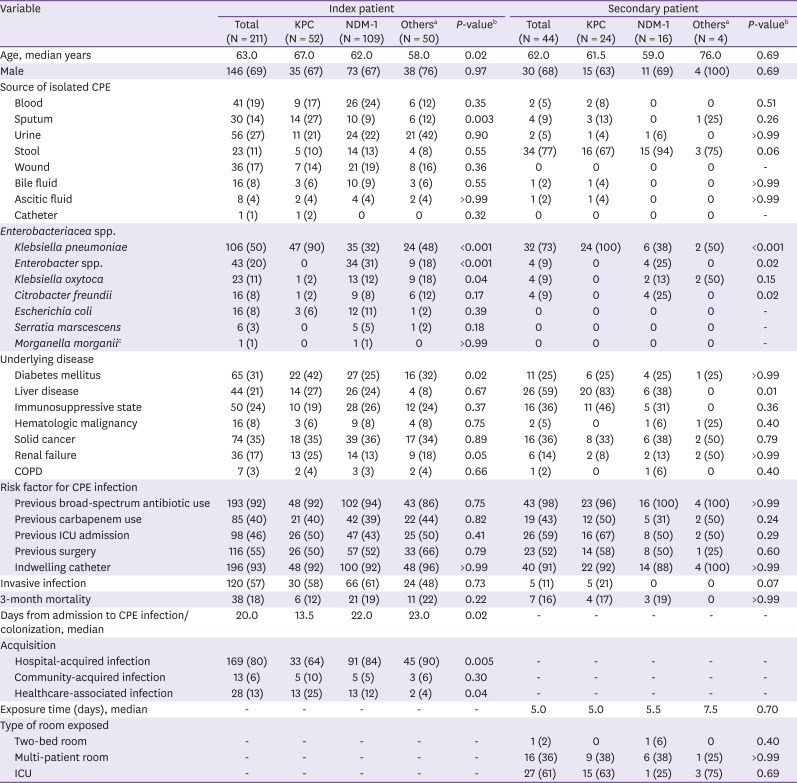
2. Clinical and microbiological characteristics of the index patients
Among the 211 CPE index patients, 52 patients (25%) tested positive for KPC-producing CRE, 109 patients (52%) tested positive for NDM-1-producing CRE, and 50 patients (24%) had CPE producing other types of carbapenemases (Verona integron-borne metallo-beta-lactamase (VIM) [n = 42], Oxacillin-hydrolyzing [OXA] [n = 6], and untypable [n = 2]). The median time from admission to detection of CPE infection or colonization was shorter in patients with KPC than NDM-1 (13.5
vs. 22.0 days (
P = 0.02) (
Table 1). A higher proportion of NDM-1-producing CRE (n = 91, 84%) were hospital-acquired than KPC-producing CRE (n = 33, 64%) (
P = 0.005). On the other hand, healthcare-associated infection were more frequent in patients with KPC-producing CRE than in those with NDM-1-producing CRE. (
P = 0.04).
K. pneumoniae was significantly more likely to be KPC-producing than NDM-1-producing (
P <0.001), while
Enterobacter spp. and
K. oxytoca were significantly more likely to be NDM-1-producing than KPC-producing (
P <0.001 and
P = 0.04, respectively).
Table 1
Baseline characteristics of the Carbapenemase-producing Enterobacteriacea (CPE) patients according to carbapenemase type among index patients or secondary patients
|
Variable |
Index patient |
Secondary patient |
|
Total (N = 211) |
KPC (N = 52) |
NDM-1 (N = 109) |
Othersa (N = 50) |
P-valueb
|
Total (N = 44) |
KPC (N = 24) |
NDM-1 (N = 16) |
Othersa (N = 4) |
P-valueb
|
|
Age, median years |
63.0 |
67.0 |
62.0 |
58.0 |
0.02 |
62.0 |
61.5 |
59.0 |
76.0 |
0.69 |
|
Male |
146 (69) |
35 (67) |
73 (67) |
38 (76) |
0.97 |
30 (68) |
15 (63) |
11 (69) |
4 (100) |
0.69 |
|
Source of isolated CPE |
|
|
|
|
|
|
|
|
|
|
|
Blood |
41 (19) |
9 (17) |
26 (24) |
6 (12) |
0.35 |
2 (5) |
2 (8) |
0 |
0 |
0.51 |
|
Sputum |
30 (14) |
14 (27) |
10 (9) |
6 (12) |
0.003 |
4 (9) |
3 (13) |
0 |
1 (25) |
0.26 |
|
Urine |
56 (27) |
11 (21) |
24 (22) |
21 (42) |
0.90 |
2 (5) |
1 (4) |
1 (6) |
0 |
>0.99 |
|
Stool |
23 (11) |
5 (10) |
14 (13) |
4 (8) |
0.55 |
34 (77) |
16 (67) |
15 (94) |
3 (75) |
0.06 |
|
Wound |
36 (17) |
7 (14) |
21 (19) |
8 (16) |
0.36 |
0 |
0 |
0 |
0 |
- |
|
Bile fluid |
16 (8) |
3 (6) |
10 (9) |
3 (6) |
0.55 |
1 (2) |
1 (4) |
0 |
0 |
>0.99 |
|
Ascitic fluid |
8 (4) |
2 (4) |
4 (4) |
2 (4) |
>0.99 |
1 (2) |
1 (4) |
0 |
0 |
>0.99 |
|
Catheter |
1 (1) |
1 (2) |
0 |
0 |
0.32 |
0 |
0 |
0 |
0 |
- |
|
Enterobacteriacea spp. |
|
|
|
|
|
|
|
|
|
|
|
Klebsiella pneumoniae
|
106 (50) |
47 (90) |
35 (32) |
24 (48) |
<0.001 |
32 (73) |
24 (100) |
6 (38) |
2 (50) |
<0.001 |
|
Enterobacter spp. |
43 (20) |
0 |
34 (31) |
9 (18) |
<0.001 |
4 (9) |
0 |
4 (25) |
0 |
0.02 |
|
Klebsiella oxytoca
|
23 (11) |
1 (2) |
13 (12) |
9 (18) |
0.04 |
4 (9) |
0 |
2 (13) |
2 (50) |
0.15 |
|
Citrobacter freundii
|
16 (8) |
1 (2) |
9 (8) |
6 (12) |
0.17 |
4 (9) |
0 |
4 (25) |
0 |
0.02 |
|
Escherichia coli
|
16 (8) |
3 (6) |
12 (11) |
1 (2) |
0.39 |
0 |
0 |
0 |
0 |
- |
|
Serratia marscescens
|
6 (3) |
0 |
5 (5) |
1 (2) |
0.18 |
0 |
0 |
0 |
0 |
- |
|
Morganella morganiic
|
1 (1) |
0 |
1 (1) |
0 |
>0.99 |
0 |
0 |
0 |
0 |
- |
|
Underlying disease |
|
|
|
|
|
|
|
|
|
|
|
Diabetes mellitus |
65 (31) |
22 (42) |
27 (25) |
16 (32) |
0.02 |
11 (25) |
6 (25) |
4 (25) |
1 (25) |
>0.99 |
|
Liver disease |
44 (21) |
14 (27) |
26 (24) |
4 (8) |
0.67 |
26 (59) |
20 (83) |
6 (38) |
0 |
0.01 |
|
Immunosuppressive state |
50 (24) |
10 (19) |
28 (26) |
12 (24) |
0.37 |
16 (36) |
11 (46) |
5 (31) |
0 |
0.36 |
|
Hematologic malignancy |
16 (8) |
3 (6) |
9 (8) |
4 (8) |
0.75 |
2 (5) |
0 |
1 (6) |
1 (25) |
0.40 |
|
Solid cancer |
74 (35) |
18 (35) |
39 (36) |
17 (34) |
0.89 |
16 (36) |
8 (33) |
6 (38) |
2 (50) |
0.79 |
|
Renal failure |
36 (17) |
13 (25) |
14 (13) |
9 (18) |
0.05 |
6 (14) |
2 (8) |
2 (13) |
2 (50) |
>0.99 |
|
COPD |
7 (3) |
2 (4) |
3 (3) |
2 (4) |
0.66 |
1 (2) |
0 |
1 (6) |
0 |
0.40 |
|
Risk factor for CPE infection |
|
|
|
|
|
|
|
|
|
|
|
Previous broad-spectrum antibiotic use |
193 (92) |
48 (92) |
102 (94) |
43 (86) |
0.75 |
43 (98) |
23 (96) |
16 (100) |
4 (100) |
>0.99 |
|
Previous carbapenem use |
85 (40) |
21 (40) |
42 (39) |
22 (44) |
0.82 |
19 (43) |
12 (50) |
5 (31) |
2 (50) |
0.24 |
|
Previous ICU admission |
98 (46) |
26 (50) |
47 (43) |
25 (50) |
0.41 |
26 (59) |
16 (67) |
8 (50) |
2 (50) |
0.29 |
|
Previous surgery |
116 (55) |
26 (50) |
57 (52) |
33 (66) |
0.79 |
23 (52) |
14 (58) |
8 (50) |
1 (25) |
0.60 |
|
Indwelling catheter |
196 (93) |
48 (92) |
100 (92) |
48 (96) |
>0.99 |
40 (91) |
22 (92) |
14 (88) |
4 (100) |
>0.99 |
|
Invasive infection |
120 (57) |
30 (58) |
66 (61) |
24 (48) |
0.73 |
5 (11) |
5 (21) |
0 |
0 |
0.07 |
|
3-month mortality |
38 (18) |
6 (12) |
21 (19) |
11 (22) |
0.22 |
7 (16) |
4 (17) |
3 (19) |
0 |
>0.99 |
|
Days from admission to CPE infection/colonization, median |
20.0 |
13.5 |
22.0 |
23.0 |
0.02 |
- |
- |
- |
- |
- |
|
Acquisition |
|
|
|
|
|
|
|
|
|
|
|
Hospital-acquired infection |
169 (80) |
33 (64) |
91 (84) |
45 (90) |
0.005 |
- |
- |
- |
- |
- |
|
Community-acquired infection |
13 (6) |
5 (10) |
5 (5) |
3 (6) |
0.30 |
- |
- |
- |
- |
- |
|
Healthcare-associated infection |
28 (13) |
13 (25) |
13 (12) |
2 (4) |
0.04 |
- |
- |
- |
- |
- |
|
Exposure time (days), median |
- |
- |
- |
- |
- |
5.0 |
5.0 |
5.5 |
7.5 |
0.70 |
|
Type of room exposed |
|
|
|
|
|
|
|
|
|
|
|
Two-bed room |
- |
- |
- |
- |
- |
1 (2) |
0 |
1 (6) |
0 |
0.40 |
|
Multi-patient room |
- |
- |
- |
- |
- |
16 (36) |
9 (38) |
6 (38) |
1 (25) |
>0.99 |
|
ICU |
- |
- |
- |
- |
- |
27 (61) |
15 (63) |
1 (25) |
3 (75) |
0.69 |
Some index cases were identified through stool collected for active surveillance cultures during a CPE outbreak. The transmission rate of the index cases where CPE was identified in active surveillance cultures was 17%, and the transmission rate of index cases identified outside of CPE outbreak situations was 11%; there was no significant difference between the two (P = 0.31).
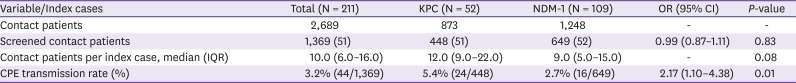
3. Comparison of the transmission capacities of KPC-producing and NDM-1-producing CRE
Of 1,369 contact patients, 44 (3.2%; 95% confidence interval 2.3-4.3%) secondary patients were identified with NDM-1-producing (16 patients) and KPC-producing (24 patients) CPE (Four patients were identified other types of carbapenemase - VIM, OXA or untypable). The median exposure time was 5 days (IQR 3.0 - 10.0) (
Table 1). The CPE acquisition rate from exposure to KPC was significantly higher than from exposure to NDM-1 (5.4%
vs. 2.7%, respectively;
P = 0.01) (
Table 2). The presence of KPC-producing CPE in index patients was an independent risk factor for transmission, while NDM-1-producing CPE was not associated with risk of transmission (
P = 0.04 and
P = 0.54, respectively) (
Table 3).
Table 2
Comparisons of the transmission frequencies of Klebsiella pneumoniae carbapenemase (KPC)- and New Delhi metallo-beta-lactamase (NDM-1)- carbapenemase producing enterobacteriaceae (CPE)
|
Variable/Index cases |
Total (N = 211) |
KPC (N = 52) |
NDM-1 (N = 109) |
OR (95% CI) |
P-value |
|
Contact patients |
2,689 |
873 |
1,248 |
- |
- |
|
Screened contact patients |
1,369 (51) |
448 (51) |
649 (52) |
0.99 (0.87–1.11) |
0.83 |
|
Contact patients per index case, median (IQR) |
10.0 (6.0–16.0) |
12.0 (9.0–22.0) |
9.0 (5.0–15.0) |
- |
0.08 |
|
CPE transmission rate (%) |
3.2% (44/1,369) |
5.4% (24/448) |
2.7% (16/649) |
2.17 (1.10–4.38) |
0.01 |
Table 3
Comparison of the clinical and microbiological characteristics of the carbapenemase producing Enterobacteriaceae (CPE) infected/colonized index cases as a function of their transmission to other patients
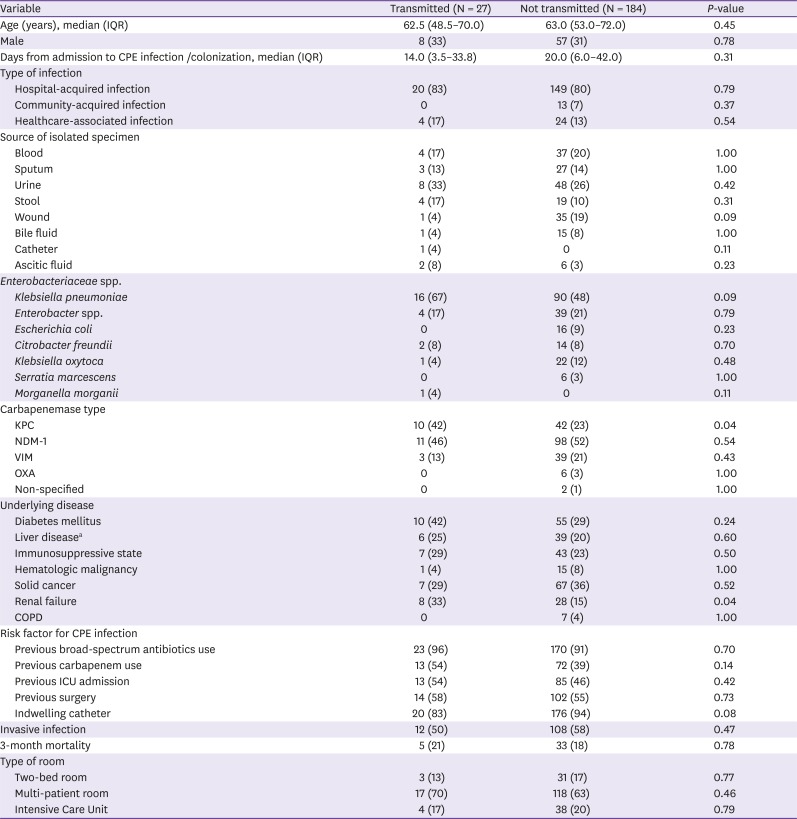
|
Variable |
Transmitted (N = 27) |
Not transmitted (N = 184) |
P-value |
|
Age (years), median (IQR) |
62.5 (48.5–70.0) |
63.0 (53.0–72.0) |
0.45 |
|
Male |
8 (33) |
57 (31) |
0.78 |
|
Days from admission to CPE infection /colonization, median (IQR) |
14.0 (3.5–33.8) |
20.0 (6.0–42.0) |
0.31 |
|
Type of infection |
|
|
|
|
Hospital-acquired infection |
20 (83) |
149 (80) |
0.79 |
|
Community-acquired infection |
0 |
13 (7) |
0.37 |
|
Healthcare-associated infection |
4 (17) |
24 (13) |
0.54 |
|
Source of isolated specimen |
|
|
|
|
Blood |
4 (17) |
37 (20) |
1.00 |
|
Sputum |
3 (13) |
27 (14) |
1.00 |
|
Urine |
8 (33) |
48 (26) |
0.42 |
|
Stool |
4 (17) |
19 (10) |
0.31 |
|
Wound |
1 (4) |
35 (19) |
0.09 |
|
Bile fluid |
1 (4) |
15 (8) |
1.00 |
|
Catheter |
1 (4) |
0 |
0.11 |
|
Ascitic fluid |
2 (8) |
6 (3) |
0.23 |
|
Enterobacteriaceae spp. |
|
|
|
|
Klebsiella pneumoniae
|
16 (67) |
90 (48) |
0.09 |
|
Enterobacter spp. |
4 (17) |
39 (21) |
0.79 |
|
Escherichia coli
|
0 |
16 (9) |
0.23 |
|
Citrobacter freundii
|
2 (8) |
14 (8) |
0.70 |
|
Klebsiella oxytoca
|
1 (4) |
22 (12) |
0.48 |
|
Serratia marcescens
|
0 |
6 (3) |
1.00 |
|
Morganella morganii
|
1 (4) |
0 |
0.11 |
|
Carbapenemase type |
|
|
|
|
KPC |
10 (42) |
42 (23) |
0.04 |
|
NDM-1 |
11 (46) |
98 (52) |
0.54 |
|
VIM |
3 (13) |
39 (21) |
0.43 |
|
OXA |
0 |
6 (3) |
1.00 |
|
Non-specified |
0 |
2 (1) |
1.00 |
|
Underlying disease |
|
|
|
|
Diabetes mellitus |
10 (42) |
55 (29) |
0.24 |
|
Liver diseasea
|
6 (25) |
39 (20) |
0.60 |
|
Immunosuppressive state |
7 (29) |
43 (23) |
0.50 |
|
Hematologic malignancy |
1 (4) |
15 (8) |
1.00 |
|
Solid cancer |
7 (29) |
67 (36) |
0.52 |
|
Renal failure |
8 (33) |
28 (15) |
0.04 |
|
COPD |
0 |
7 (4) |
1.00 |
|
Risk factor for CPE infection |
|
|
|
|
Previous broad-spectrum antibiotics use |
23 (96) |
170 (91) |
0.70 |
|
Previous carbapenem use |
13 (54) |
72 (39) |
0.14 |
|
Previous ICU admission |
13 (54) |
85 (46) |
0.42 |
|
Previous surgery |
14 (58) |
102 (55) |
0.73 |
|
Indwelling catheter |
20 (83) |
176 (94) |
0.08 |
|
Invasive infection |
12 (50) |
108 (58) |
0.47 |
|
3-month mortality |
5 (21) |
33 (18) |
0.78 |
|
Type of room |
|
|
|
|
Two-bed room |
3 (13) |
31 (17) |
0.77 |
|
Multi-patient room |
17 (70) |
118 (63) |
0.46 |
|
Intensive Care Unit |
4 (17) |
38 (20) |
0.79 |
Discussion
We found that the transmission of CPE from index cases to patients in close contact was significantly affected by CPE type: KPC-producing CRE were twice as likely to be transmitted as NDM-1-producing CRE. We have seen few other reports comparing the transmission rates of KPC- and NDM-1-producing organisms. Therefore, our finding provides important insight into the epidemiology of hospital transmission of KPC- and NDM-1-producing organisms.
Among the index cases, most of the KPC-producing CRE organisms were
K. pneumoniae, while the NDM-1-producing CRE organisms were a mixture of
Enterobacteriaceae. Many
K. pneumoniae infections can be acquired in hospital and healthcare settings [
9]. KPC-producing CRE have been described as “super-spreaders” due to their higher transmission rates; we have previously reported on the lower probability of clearance of KPC-producing strains compared to non-KPC-producing strains [
1011]. NDM-1-producing organisms may have transmission routes other than via direct contact [
12], for example, NDM-1-producing organisms might be transmitted from a contaminated plumbing fixtures such as the P-trap of a sink or shower [
13]. Therefore contact tracing based on proximity to the index patients may miss such secondary cases.
A study conducted by Schwartz-Neiderman et al. reported that 40 index cases were in contact with 735 others, of whom 53 cases (7.2%) were newly-diagnosed with CPE [
14]. It is worth noting that only 3.2% (95% CI, 2.3 - 4.3) patients in our study were found to have acquired CPE during surveillance of patients who had been in close contact with index patients. The reason for this different transmission rate is unclear. The relatively low transmission rate in our study might raise questions about the need for contact tracing for index patients with CPE-producing organisms. However, our findings suggest that a cautious approach to KPC-producing CRE is warranted.
Our study had several limitations. First, we did not consider compliance with hand hygiene or contact precautions including the use of personal protective equipment by medical staff at ordinary times; before CPE patients were detected. Second, we could not perform whole genome sequencing to confirm the epidemiological data for the CPE-infected or colonized patients; it could not confirm the transmission from the index patients to the infected/colonized patients identified through surveillance. Because of this, epidemiological linkages are presented as estimates based on duration of admission and the hospital wards to which patients were admitted. Third, about half of the contact patients were not screened for CPE due to early discharge or death. Among the missing cases (1,320 cases), 896 cases (68%) had early discharge, and 424 cases (32%) had died. The patients were at less risk for transmission in the hospital because the medical problem was resolved and discharged, which may have introduced some selection bias. However, this potential selection bias reflects the fact that follow-up after surveillance screenings for CPE close contact patients in a clinical setting is difficult. Fourth, we could not be certain about “true” secondary cases because many factors contribute to transmission of multidrug-resistant organisms, such as environmental contaminations. Fifth, we cannot be certain that close contact patients were not infected/colonized with CRE before the contact with index patients. Finally, we could not perform the multivariate analysis for identifying independent risk factors for the transmission events due to low event numbers. So it is difficult to draw a firm conclusion that K. pneumonia itself instead of KPC-producing organisms may contribute to the high transmission events.
In conclusion, CPE transmission rates differed between the carbapenemase types. KPC- producing CRE were transmitted twice as frequently as NDM-1-producing CRE.
ACKNOWLEDGEMENT
We thank members of the Asan Medical Center infection control team for collection of patient clinical data. Moreover, we also thank all the physicians and medical staff who worked to manage infection control in CPE patients.
References
1. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017; 215(suppl_1):S28–S36. PMID:
28375512.

2. Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010; 362:1804–1813. PMID:
20463340.

3. Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. JAMA. 2016; 316:1193–1204. PMID:
27654605.

4. Nordmann P, Cuzon G, Naas T. The real threat of
Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009; 9:228–236. PMID:
19324295.
5. Lee E, Lee S, Park H, Kim S, Lee H. Analysis of carbapenemase-producing Enterobacteriaceae (CPE) surveillance results for 2017 in Korea: Comparison with the surveillance results of the previous 5 years (2012-2016). PHWR. 2018; 11:1586–1594.
6. Cardoso T, Almeida M, Friedman ND, Aragão I, Costa-Pereira A, Sarmento AE, Azevedo L. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med. 2014; 12:40. PMID:
24597462.

7. Korean Society for Healthcare-associated Infection Control and Prevention (KOSHIC). Korean national healthcare-associated infections surveillance system manual. 2018.
8. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: M100. 27th ed. Wayne, PA: CLSI;2017.
9. Podschun R, Ullmann U.
Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998; 11:589–603. PMID:
9767057.
10. Lerner A, Adler A, Abu-Hanna J, Cohen Percia S, Kazma Matalon M, Carmeli Y. Spread of KPC-producing carbapenem-resistant Enterobacteriaceae: the importance of super-spreaders and rectal KPC concentration. Clin Microbiol Infect. 2015; 21:470.e1–470.e7.

11. Lim YJ, Park HY, Lee JY, Kwak SH, Kim MN, Sung H, Kim SH, Choi SH. Clearance of carbapenemase-producing Enterobacteriaceae (CPE) carriage: a comparative study of NDM-1 and KPC CPE. Clin Microbiol Infect. 2018; 24:1104.e5–1104.e8.
12. Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis. 2011; 11:355–362. PMID:
21478057.

13. Seara N, Oteo J, Carrillo R, Pérez-Blanco V, Mingorance J, Gómez-Gil R, Herruzo R, Pérez-Vázquez M, Astray J, García-Rodríguez J, Ruiz-Velasco LM, Campos J, de Burgos C, Ruiz-Carrascoso G. Interhospital spread of NDM-7-producing Klebsiella pneumoniae belonging to ST437 in Spain. Int J Antimicrob Agents. 2015; 46:169–173. PMID:
25982912.

14. Schwartz-Neiderman A, Braun T, Fallach N, Schwartz D, Carmeli Y, Schechner V. Risk factors for carbapenemase-producing carbapenem-resistant Enterobacteriaceae (CP-CRE) acquisition among contacts of newly diagnosed CP-CRE patients. Infect Control Hosp Epidemiol. 2016; 37:1219–1225. PMID:
27452597.








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download