Abstract
Purpose
Diabetic nephropathy is one of the most important diabetic complications prompted by chronic hyperglycemia, characterized by glomerulosclerosis, tubular fibrosis, and it eventually causes kidney failure. Nobiletin is a polymethoxyflavone present in tangerine and other citrus peels, and has anti-cancer and anti-inflammatory effects. This study investigated the effects of nobiletin on glomerular fibrosis through inhibition of the transforming growth factor (TGF)-β1-Src-caveolin-1 pathway.
Methods
Human renal mesangial cells (HRMC) were incubated in media containing 33 mM glucose with or without 1–20 uM nobiletin for 3 day. The cellular expression levels of fibrogenic collagen IV, fibronectin, connective tissue growth factor (CTGF), TGF-β1, Src and caveolin-1 were all examined. In addition, TGF-β1, Src and caveolin-1 proteins were screened to reveal the relationship among TGF-β1-Src-caveolin-1 signaling in glomerular fibrosis.
Results
High glucose promoted the production of collagen IV, fibronectin and CTGF in HRMC, which was inhibited in a dose dependent manner by 1–20 uM nobiletin. The Western blot data showed that high glucose elevated the expression of TGF-β1, Src, caveolin-1 and Rho GTPase. When nobiletin was treated to the HRMC exposed to high glucose, the expression of TGF-β1-Src-caveolin-1 was dampened. Finally, TGF-β1-Src-caveolin-1 signaling pathway was activated in high glucose-exposed HRMC, and such activation was encumbered by nobiletin.
Conclusion
These result demonstrated that nobiletin blunted high glucose-induced extracellular matrix accumulation via inhibition of the TGF-β1-Src-caveolin-1 related intracellular signaling pathway. Nobiletin may be a potent renoprotective agent to counteract diabetes-associated glomerular fibrosis that leads to kidney failure.
References
1. Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008; 4(3):575–596.
2. Schena FP, Gesualdo L. Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol. 2005; 16(Suppl 1):S30–S33.

3. Pozzi A, Voziyan PA, Hudson BG, Zent R. Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr Pharm Des. 2009; 15(12):1318–1333.

4. Fukuda N, Tahira Y, Matsuda H, Matsumoto K. Transforming growth factor-beta as a treatment target in renal diseases. J Nephrol. 2009; 22(6):708–715.
5. Li J, Lim SS, Lee JY, Kim JK, Kang SW, Kim JL, et al. Purple corn anthocyanins dampened high-glucose-induced mesangial fibrosis and inflammation: possible renoprotective role in diabetic nephropathy. J Nutr Biochem. 2012; 23(4):320–331.

6. Ungefroren H, Witte D, Lehnert H. The role of small GTPases of the Rho/Rac family in TGF-β-induced EMT and cell motility in cancer. Dev Dyn. 2018; 247(3):451–461.

7. Li J, Lim SS, Lee ES, Gong JH, Shin D, Kang IJ, et al. Isoangustone A suppresses mesangial fibrosis and inflammation in human renal mesangial cells. Exp Biol Med (Maywood). 2011; 236(4):435–444.

8. Peng F, Wu D, Gao B, Ingram AJ, Zhang B, Chorneyko K, et al. RhoA/Rho-kinase contribute to the pathogenesis of diabetic renal disease. Diabetes. 2008; 57(6):1683–1692.

9. Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-β/SMAD signaling through an interaction with the TGF-β type I receptor. J Biol Chem. 2001; 276(9):6727–6738.

10. Van Krieken R, Krepinsky JC. Caveolin-1 in the pathogenesis of diabetic nephropathy: potential therapeutic target? Curr Diab Rep. 2017; 17(3):19.

11. Lu Y, Tang L, Li Y, He Q. High glucose-induced fibronectin upregulation in cultured mesangial cells involves caveolin-1-dependent RhoA-GTP activation via Src kinase. Mol Med Rep. 2016; 14(1):963–968.

12. Youn K, Lee S, Jun M. Discovery of nobiletin from citrus peel as a potent inhibitor of β-amyloid peptide toxicity. Nutrients. 2019; 11(11):2648.

13. Huang H, Li L, Shi W, Liu H, Yang J, Yuan X, et al. The multifunctional effects of nobiletin and its metabolites in vivo and in vitro. Evid Based Complement Alternat Med. 2016; 2016:2918796.
14. Wilson HM, Stewart KN. Glomerular epithelial and mesangial cell culture and characterization. Methods Mol Biol. 2012; 806:187–201.

15. Li J, Kang SW, Kim JL, Sung HY, Kwun IS, Kang YH. Isoliquiritigenin entails blockade of TGF-β1-SMAD signaling for retarding high glucose-induced mesangial matrix accumulation. J Agric Food Chem. 2010; 58(5):3205–3212.

16. Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008; 233(1):4–11.

Fig. 1.
Chemical structure of nobiletin (A), and cell viability (B, C) and collagen IV secretion (C) and cellular expression (D) of HRMC challenged with 33 mM glucose in the absence and presence of nobiletin. HRMC were treated with 1–20 µM nobiletin for 3 days in the culture media of 33 mM glucose. Nobiletin was dissolved DMSO as stock solution and then dilute with culture media. Cells were also incubated with 5.5 mM glucose and 27.5 mM mannitol as osmotic controls. For the secretion of collagen IV (C), culture media were subjected to western blot analysis with a primary antibody against collagen IV. Immunocytochemical analysis (D) was performed for cellular collagen IV localization visualized with Cy3-conjugated anti-rabbit IgG and nuclear counterstaining was done with DAPI. Magnification, 400-fold. The bar graphs (means ± SEM, n = 3) in the bottom panel represent quantitative results obtained from a densitometer. Values not sharing a common letter are significantly different at p < 0.05. HRMC, human renal mesangial cells; DMSO, dimethyl sulfoxide; IgG, immunoglobulin G; DAPI, 4′,6-diamidino-2-phenylindole; SEM, standard error of the mean.
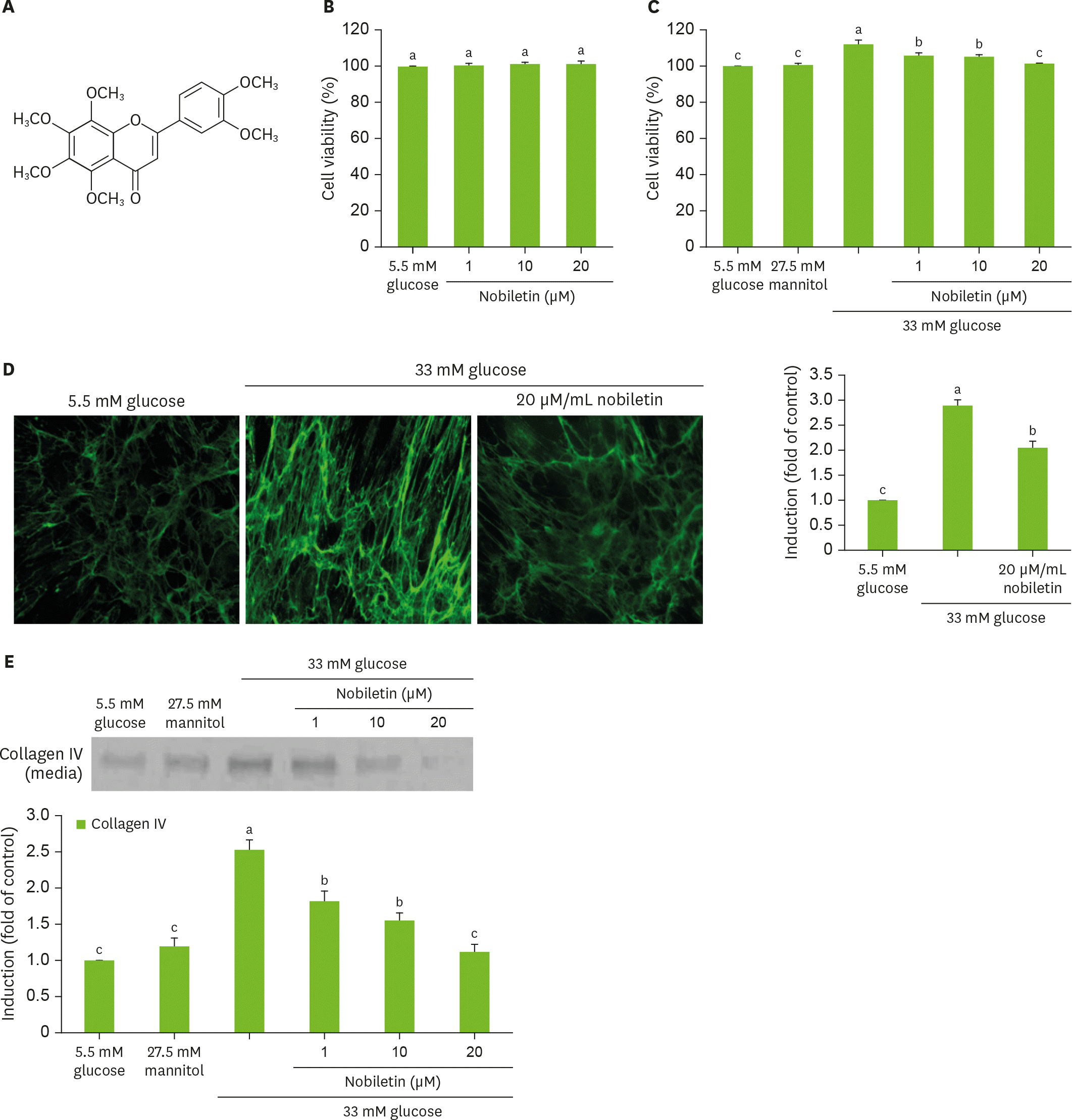
Fig. 2.
Temporal induction of fibronectin CTGF by high glucose (A), their inhibition by nobiletin (B), and immunocytochemial staining (C) showing inhibition of fibronectin induction by nobiletin. HRMC were treated with 1–20 µM nobiletin for 3 days in the culture media of 33 mM glucose. Cells were also incubated in 5.5 mM glucose and 27.5 mM mannitol as osmotic controls. Cell lysates were subject to western blot analysis with a primary antibody against fibronectin and CTGF (A, B). β-actin protein was used as an internal control. The bar graphs (means ± SEM, n = 3) in the bottom panels represent quantitative results of blots. Values not sharing a common letter are significantly different at p < 0.05. Fibronectin was visualized by staining with a green FITC-conjugated IgG (magnification, 400-fold). CTGF, connective tissue growth factor; SEM, standard error of the mean; FITC, fluorescein isothiocyanate; IgG, immunoglobulin G.
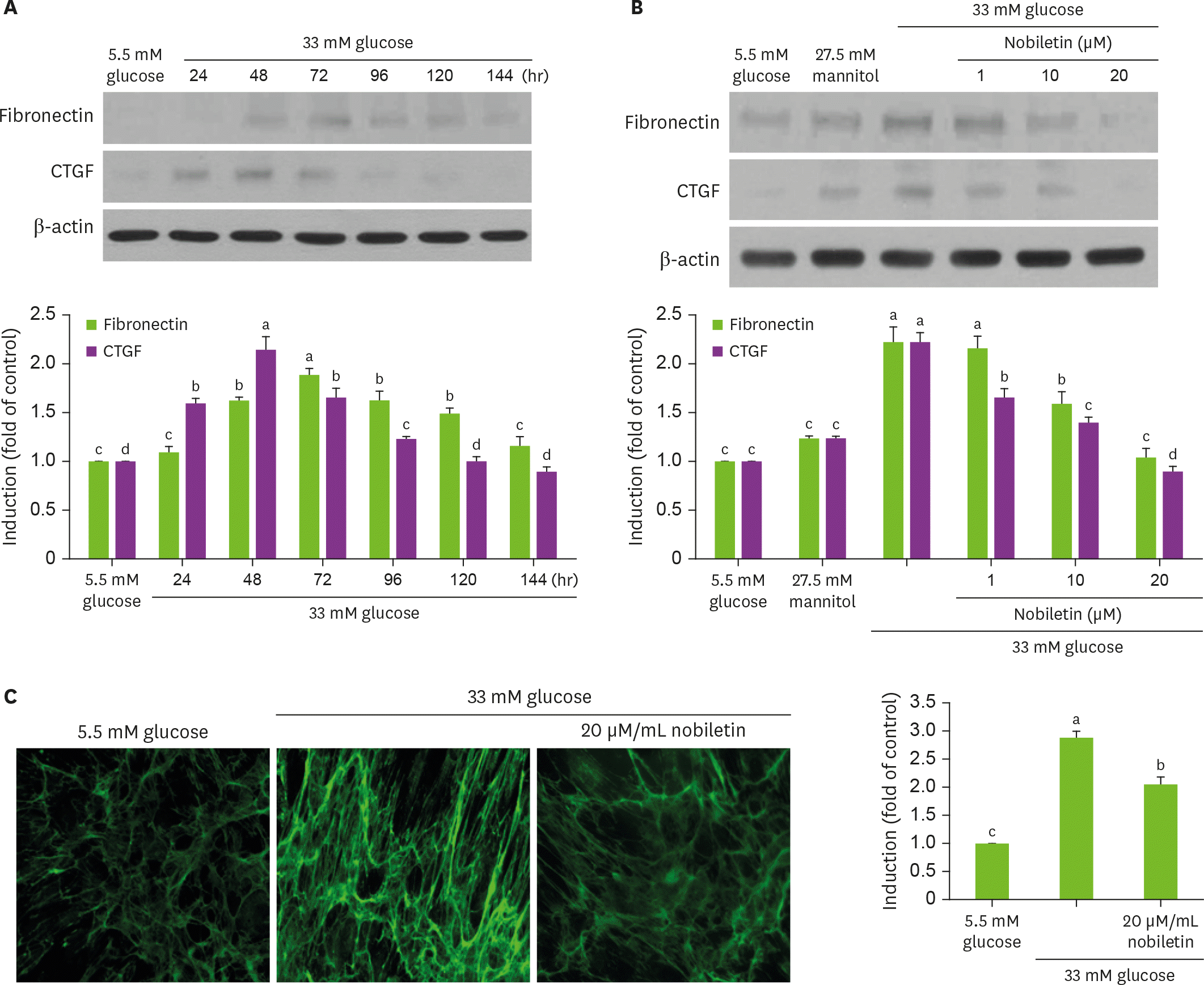
Fig. 3.
Temporal induction of Src and caveolin-1 by high glucose (A) and their inhibition by nobiletin (B) and western blot data (C) showing inhibition of RhoA and ROCK induction by nobiletin. HRMC were treated with 1–20 µM nobiletin for 3 days in the culture media of 33 mM glucose. Cells were also incubated in 5.5 mM glucose and 27.5 mM mannitol as osmotic controls. Cell lysates were subject to western blot analysis with a primary antibody against Src, caveolin-1, RhoA and ROCK (A, B). β-actin protein was used as an internal control. The bar graphs (means ± SEM, n = 3) in the bottom panels represent quantitative results of blots. Values not sharing a common letter are significantly different at p < 0.05. RhoA, Ras homolog family member A; ROCK, Rho-associated protein kinase; SEM, standard error of the mean.
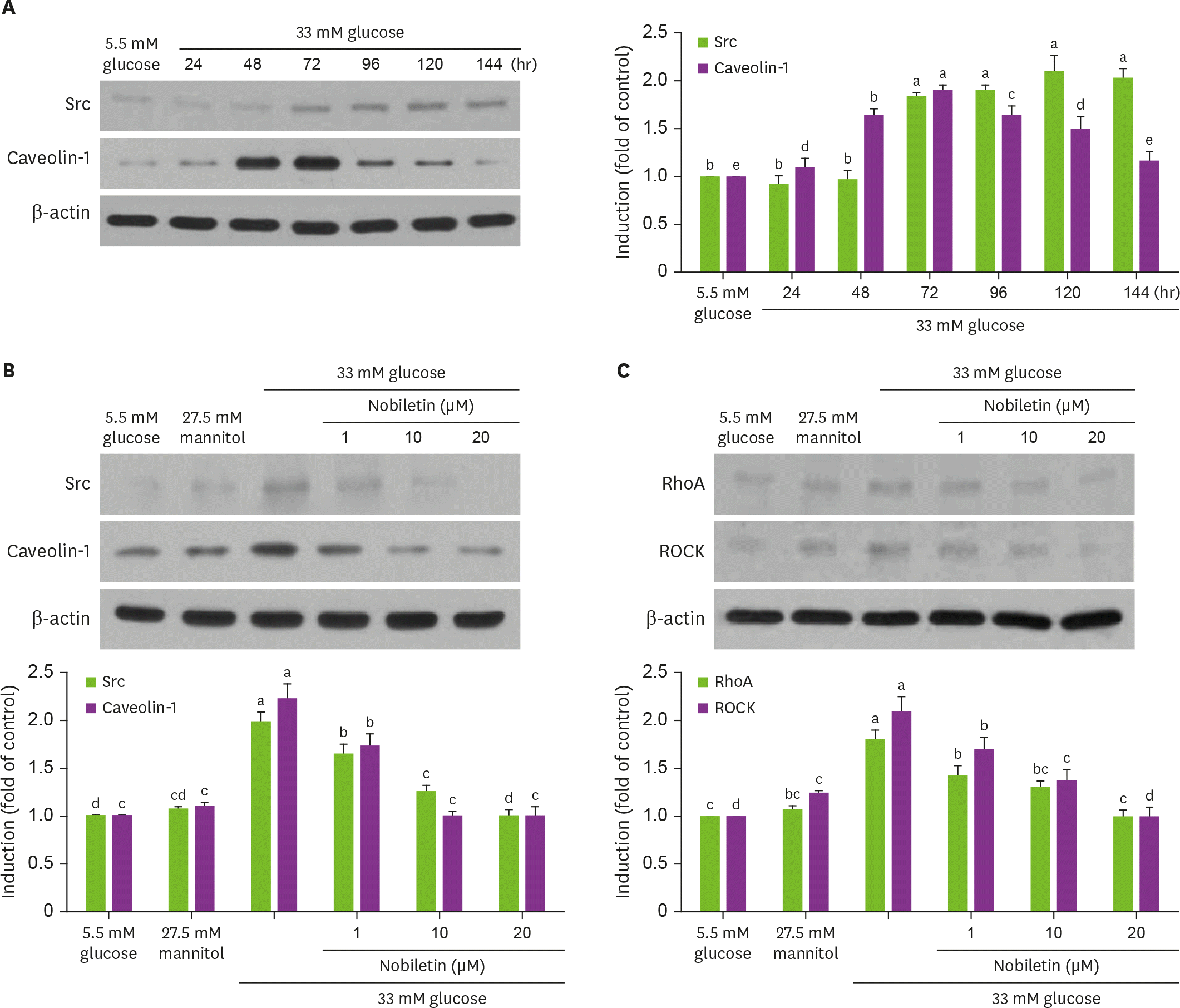
Fig. 4.
Effect of nobiletin on TGF-β1, TGF-β1 receptor I and II induction (A). HRMC were treated with 1–20 µM nobiletin for 3 days in the culture media of 33 mM glucose. Cells were also incubated in 5.5 mM glucose and 27.5 mM mannitol as osmotic controls. In other experiments, cells were cultured with 10 ng/mL TGF-β1 absence and presence of 20 µM nobiletin for 3 day (B). Cell lysates were subject to 8%–12% SDS-PAGE and western blot analysis with a primary antibody against TGF-β1, TGF-β1 receptor I and II, fibronectin, Src, caveolin-1 and RhoA (A and B). β-actin protein was used as an internal control. The bar graphs (mean ± SEM, n = 3) in the bottom panels represent quantitative results of blots. Values not sharing a common letter are significantly different at p < 0.05. TGF, transforming growth factor; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; RhoA, Ras homolog family member A; SEM, standard error of the mean.
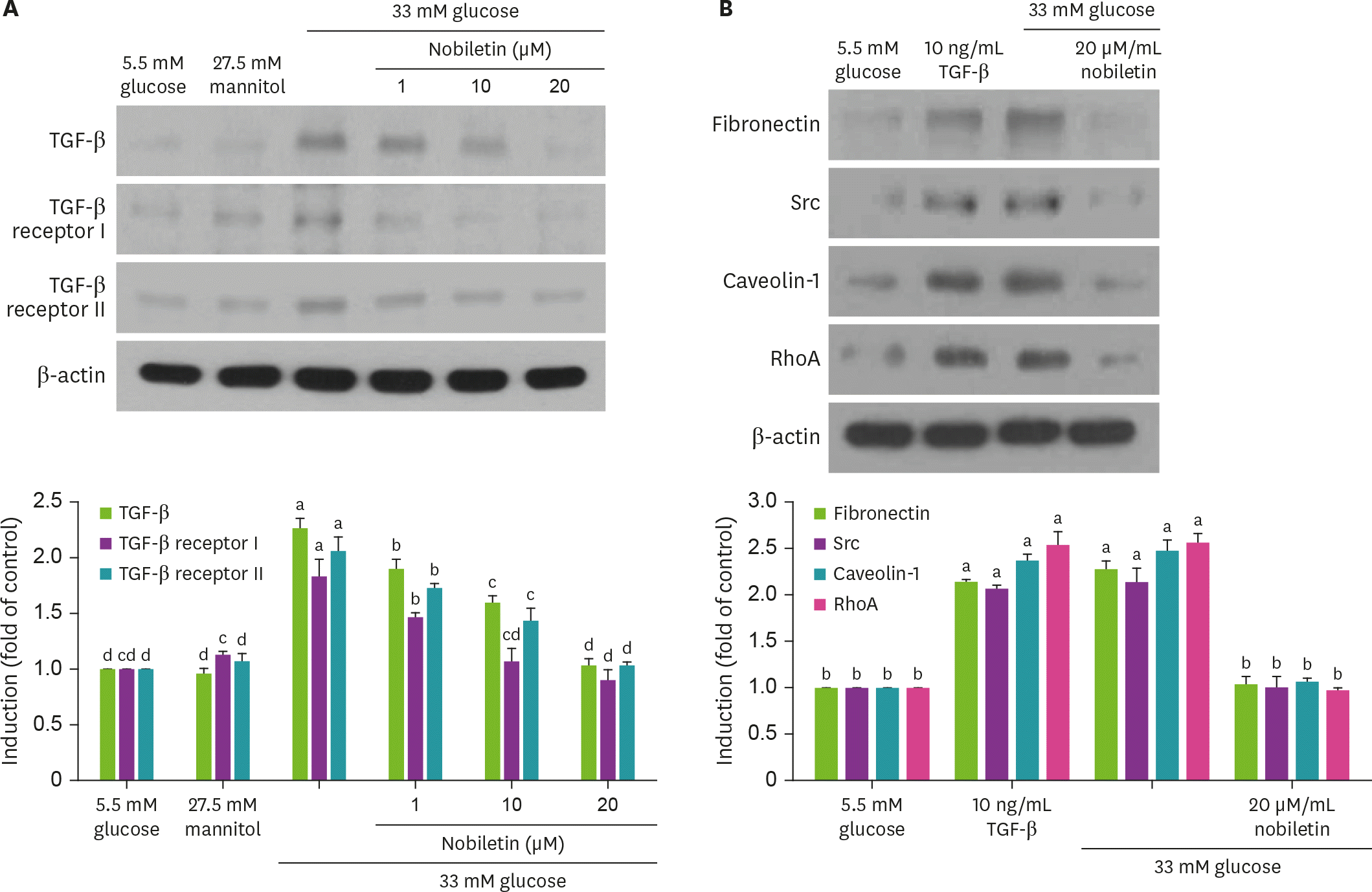
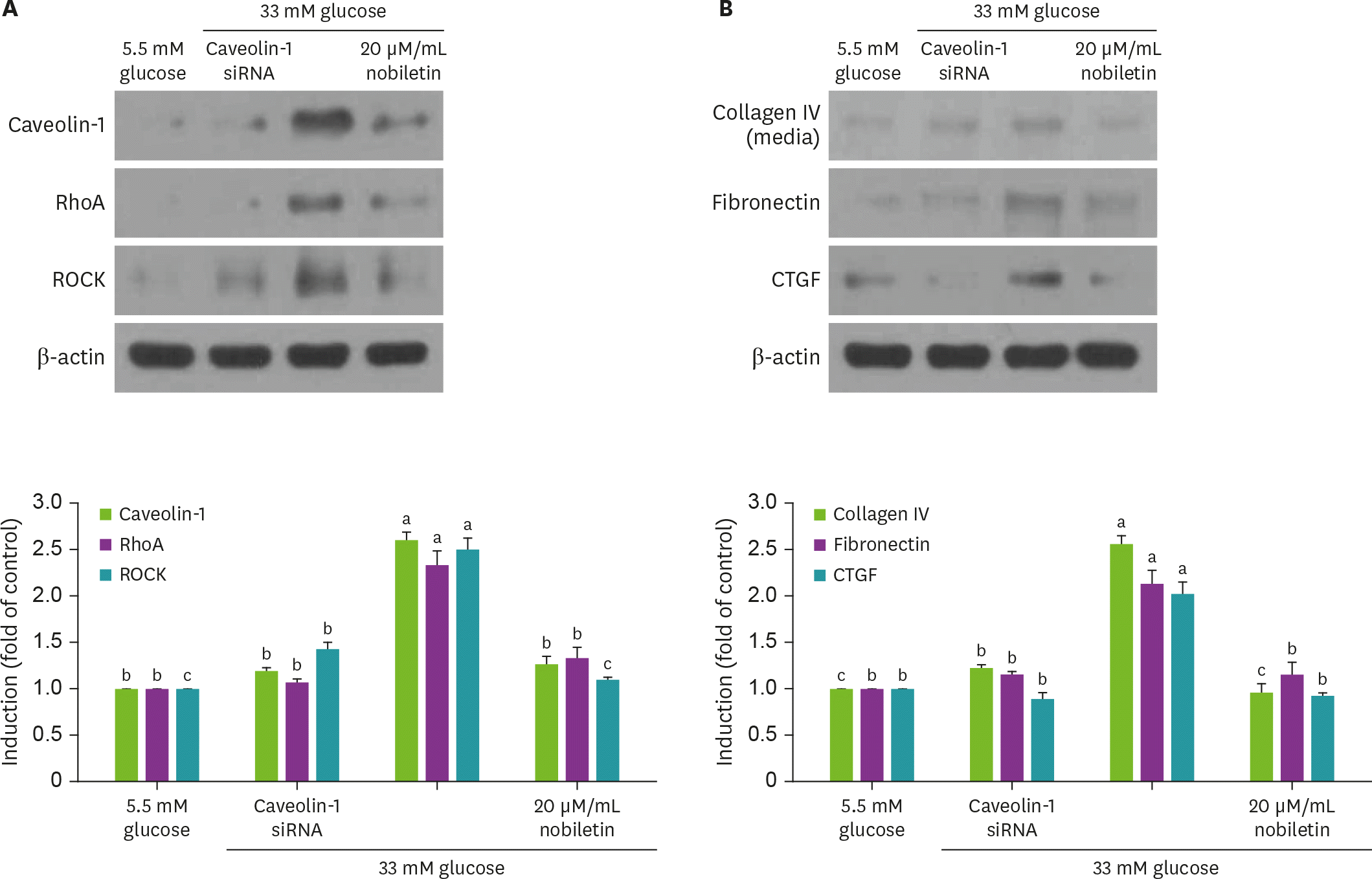
Fig. 5.
Effect of nobiletin and silencing of caveolin-1 genes on expression of caveolin-1, RhoA, ROCK, collagen IV, fibronectin or CTGF in the presence of 33 mM glucose (A and B). Caveolin-1 siRNA-transfected HRMC were incubated in 33 mM glucose for 3 day. Cell lysates were subject to 8%–12% western blot analysis with a primary antibody against caveolin-1, RhoA, ROCK, collagen IV, fibronectin or CTGF. β-actin protein was used as an internal control. The bar graphs (mean ± SEM, n = 3) in the bottom panels represent quantitative results of blots. Values not sharing a common letter are significantly different at p < 0.05. RhoA, Ras homolog family member A; ROCK, Rho-associated protein kinase; CTGF, connective tissue growth factor; siRNA, small interfering RNA; HRMC, human renal mesangial cells; SEM, standard error of the mean.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download