Abstract
Currently, there has been rapid increase in the studies about salivary production because of the hyposalivation, xerostomia, caused by radiotherapy for head and neck cancer, Sjogren syndrome and aging. An overview of anatomy and development of salivary gland is crucial to understand about the patho-physiological disorders related with saliva. For study of the morphogenesis and development of salivary glands, experiment using rodent models is widely necessary. This review wraps up the early to latest studies – the different features of each salivary gland, morphogenesis of developing salivary glands, and the comparison of human and rodent salivary glands. The goal of this review is to provide hypothesis for the further researches about differentiation of specific acinar cells, from which it is determined to be specific acini. Additionally, we discuss approaches to regenerate the function of salivary glands using environmental factor, time dependent factor and nerve factor.
References
1. Holmberg KV, Hoffman MP. Anatomy, biogenesis, and regeneration of salivary glands. Monogr Oral Sci. 2014; 24:1–13.

3. Vissink A, Mitchell JB, Baum BJ, Limesand KH, Jensen SB, Fox PC, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010; 78:983–91.

4. Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000; 131:887–99.

5. Nanci A. Salivary glands. Ten Cate's Oral Histology: Devel-pment, Structure, and Function. 8 th ed. St. Louis: Mosby;2012. pp.p. 253–77.
6. De Almeida. Vigna PD, Gregio AM, Machado MA, De Lima AA, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008; 9:72–80.
7. Zhang H, Boddupally K, Kandyba E, Kobielak K, Chen Y, Zu S, et al. Defining the localization and molecular characteristic of minor salivary gland label retaining cells. Stem Cells. 2014; 32:2267–77.
8. Sreebny L. Xerostomia: diagnosis, management and clinical complications. Saliva and Oral Health. 1996. 45–50.
10. Burlage FR, Coppes R, Meertens H, Stokman M, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol. 2001; 61:271–4.

11. Hand AR, Pathmanathan D, Field RB. Morphological features of the minor salivary glands. Arch Oral Biol. 1999; 44:S3–10.

12. Riva A, Puxeddu R, Uras L, Loy F, Serreli S, Testa Riva F. A high resolution sem study of human minor salivary glands. Eur J Morphol. 2000; 38:219–26.

13. Tabak LA, Levine MJ, Mandel ID, Ellison SA. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982; 11:1–17.

15. Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000; 11:216–29.

16. Chiego DJ, Daniel J. Essentials of Oral Histology and Embryology: A Clinical Approach. 4th ed.St. Louis: Mosby;2013. pp.p. 184–95.
17. Bialek EJ, Jakubowski W, Zajkowski P, Szopinski KT, Os-molski A. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions, and pitfalls. Radiographics. 2006; 26:745–63.

18. Abdullah A, Rivas FFR, Srinivasan A. Imaging of the Salivary Glands. Semin Roentgenol. 2013; 48:65–74.

19. Saracco CG, Crabill E. Anatomy of the human salivary glands. Biology of the salivary glands. Boca Raton: CRC Press. Inc. 1993. pp.1–14.
20. Amano O, Mizobe K, Bando Y, Sakiyama K. Anatomy and histology of rodent and human major salivary glands. Acta Histochem Cytochem. 2012; 45:241–50.
21. Jaskoll T, Melnick M. Submandibular gland morphogenesis: Stage-specific expression of TGF-α/EGF, IGF, TGF-β, TNF, and IL-6 signal transduction in normal embryonic mice and the phenotypic effects of TGF-β2, TGF-β3, and EGF-r null mutations. The Anat Rec. 1999; 256:252–68.

22. Westmuckett AD, Siefert JC, Tesiram YA, Pinson DM, Moore KL. Salivary gland hypofunction in tyrosylprotein sulfotransferase-2 knockout mice is due to primary hypothyroidism. PLoS One. 2013; 8:e71822.

23. Hauser BR, Hoffman MP. Regulatory Mechanisms Driving Salivary Gland Organogenesis. Curr Top Dev Biol. 2015; 115:111–30.

24. Harunaga J, Hsu JC, Yamada KM. Dynamics of salivary gland morphogenesis. J Dent Res. 2011; 90:1070–7.

25. Amano O. The salivary gland: anatomy for surgeons and researchers. Jpn J Oral Maxillofac Surg. 2011; 57:384–93.

26. Jonjic S. Surgical removal of mouse salivary glands. Curr Protoc Immunol. 2001 Aug.doi:. DOI: 10.1002/0471142735. im0111s43.
27. Gresik EW. The granular convoluted tubule (GCT) cell of rodent submandibular glands. Microsc Res Tech. 1994; 27:1–24.

28. Lu C, Fuchs E. Sweat Gland Progenitors in Development, Homeostasis, and Wound Repair. Cold Spring Harb Perspect Med. 2014 Feb 1.doi:. DOI: 10.1101/cshperspect.a015222.
30. Schaller M, Plewig G. Structure and function of eccrine, apocrine, apoeccrine and sebaceous glands. Dermatology. 2003; 1:525–30.
31. Eroschenko VP, Di Fiore MS. DiFiore's atlas of histology with functional correlations. Lippincott Williams & Wilkins;2013. pp.p. 451–76.
32. Junqueira LC, Carneiro J. Basic histology: text and atlas. New York: McGraw-Hill Professional;2005. pp.p. 205–22.
33. Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006; 74:349–64.

34. Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun. 2013; 4:1494.

35. Sakai T. Development and regeneration of salivary gland toward for clinical application. Oral Science International. 2016; 13:7–14.

36. Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med. 2000; 11:199–215.
37. Larsen M, Yamada KM, Musselmann K. Systems analysis of salivary gland development and disease. Wiley Interdiscip Rev Syst Biol Med. 2010; 2:670–82.

38. Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014; 25–26:52–60.

39. Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, et al. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal. 2009; 2:ra55. doi:. DOI: 10.1126/scisignal.2000304.

40. Nedvetsky PI, Emmerson E, Finley JK, Ettinger A, Cruz-Pa-checo N, Prochazka J, et al. Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell. 2014; 30:449–62.

41. Jaskoll T, Abichaker G, Witcher D, Sala FG, Bellusci S, Ha-jihosseini MK, et al. FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev Biol. 2005; 5:11.

42. Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, Witt RL, et al. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep. 2013; 1:604–19.

43. Patel N, Sharpe PT, Miletich I. Coordination of epithelial branching and slivary gland lumen formation by Wnt and FGF signals. Dev Biol. 2011; 358:156–67.
44. Patel VN, Lombaert IM, Cowherd SN, Shworak NW, Xu Y, Liu J, et al. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulation 3-O-sulfotransferases. Dev Cell. 2014; 29:662–73.
45. Steinberg Z, Myers C, Heim VM, Lathrop CA, Rebustini IT, Stewart JS, et al. FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development. 2005; 132:1223–34.

46. Häärä O, Koivisto T, Miettinen PJ. EGF-receptor regulates salivary gland branching morphogenesis by supporting proliferation and maturation of epithelial cells and survival of mesenchymal cells. Differentiation. 2009; 77:298–306.

47. Haara O, Fujimori S, Schmidt-Ullrich R, Hartmann C, Thes-leff I, Mikkola ML. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development. 2011; 138:2681–91.
48. Knosp WM, Knox SM, Lombaert IM, Haddox CL, Patel VN, Hoffman MP. Submandibular parasympathetic ganglio-genesis requires sprout-dependent Wnt signals from epithelial progenitors. Dev Cell. 2015; 32:667–77.
49. Coghlan MP, Culbert AA, Cross DAE, Corcoran SL, Yates JW, Pearce NJ, et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol. 2000; 7:793–803.

50. Jaskoll T, Leo T, Witcher D, Ormestad M, Astorga J, Bringas P Jr, et al. Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn. 2004; 229:722–32.

51. Blecher SR, Debertin M, Murphy JS. Pleiotropic effect of Tabby gene on epidermal growth factor-containing cells of mouse submandibular gland. Anat Rec. 1983; 207:25–9.

52. Jaskoll T, Zhou YM, Trump G, Melnick M. Ectodysplasin receptormediated signaling is essential for embryonic submandibular salivary gland development. Anat Rec A Discov Mol Cell Evol Biol. 2003; 271:322–31.

53. Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, et al. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development. 2002; 129:5767–78.

54. Fukuda Y, Masuda Y, Kishi J, Hashimoto Y, Hayakawa T, Nogawa H, et al. The role of interstitial collagens in cleft formation of mouse embryonic submandibular gland during initial branching. Development. 1988; 103:259–67.

55. Hayakawa T, Kishi J, Nakanishi Y. Salivary gland morphogenesis: possible involvement of collagenase. Matrix Suppl. 1992; 1:344–51.
56. Patel VN, Knox SM, Likar KM, Lathrop CA, Hossain R, Eftekhari S, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007; 134:4177–86.

57. Rebustini IT, Patel VN, Stewart JS, Layvey A, Georg-es-Labouesse E, Miner JH, et al. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol. 2007; 308:15–29.
58. Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003; 423:876–81.

59. Johns ME. The salivary glands: anatomy and embryology. Otolaryngol Clin North Am. 1977; 10:261–71.

Fig. 1.
Anatomy of the anterior neck portions of developing salivary glands of mice (a-c). Embryonic day 16.5 (E16.5), post-natal day 0 (PN0) and adult mice showing the location of parotid gland, submandibular gland and sublingual gland. Scale bars, 1 mm.
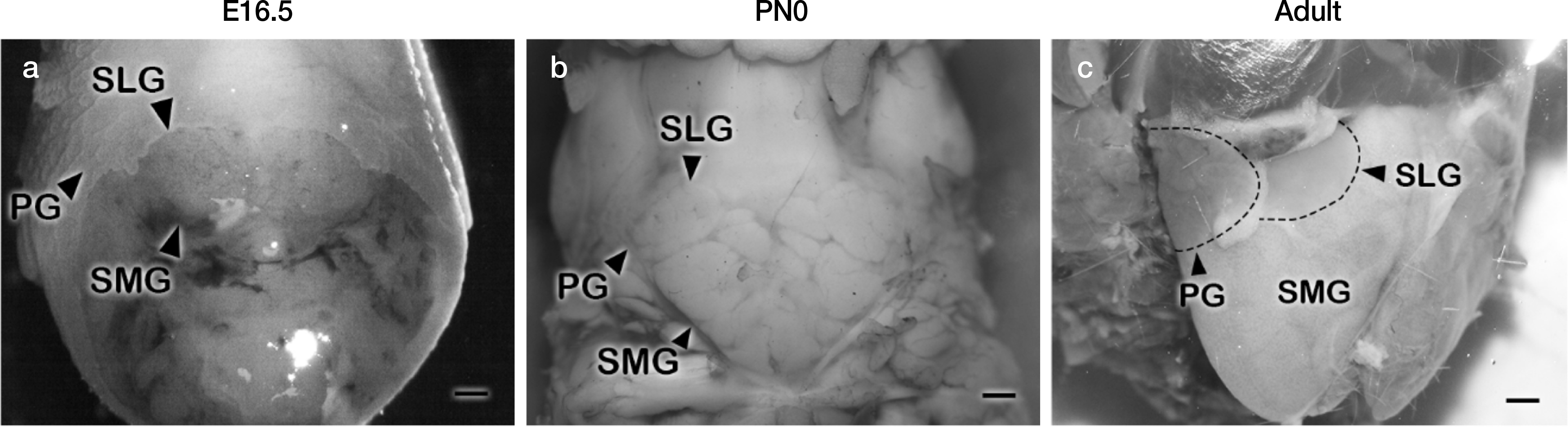
Fig. 2.
Hematoxylin and eosin staining of the developing parotid gland (PG; a, c, e) and sublingual gland (SLG; b, d, f). Embryonic day 15.5 PG showing epithelial bud (a). Post-natal day 0 (PN0) PG showing terminal epithelial buds (c). Adult PG showing dark-colored acinar cells which characterizes serous acinar cell (e). E15.5 SLG with developing epithelial end buds and lumen formation (b). PN0 showing terminal tu-bules and differentiating acinar cells (d). Adult SLG showing light-colored acinar cells which characterizes mucous acinar cells (f). TT terminal tubule, ED excretory duct, SC serous acinar cell, ME myoepithelial cell, MC mucous acinar cell.
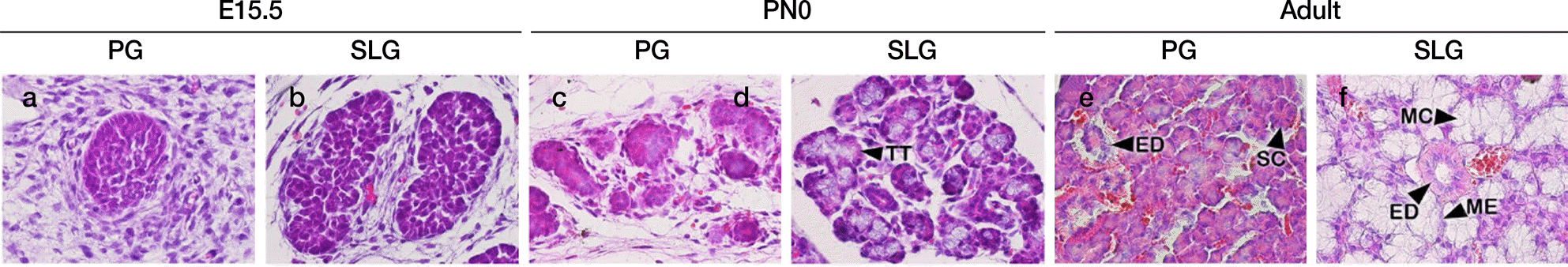
Table 1.
Functions of saliva
Table 2.
Minor salivary glands and contribution to saliva
| Name | Location | Type secretion |
|---|---|---|
| Labial | Lips | Mixed [16] |
| Buccal | Cheeks | Mixed [16] |
| Palatine | Hard and soft | Pure mucous [16] |
| Lingual | Anterior | Mixed |
| Middle | Serous | |
| Posterior | Pure mucous [16] |
Table 3.
Signaling genesis pathways involved in salivary gland morpho-
Table 4.
Characteristics of major salivary glands




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download