1. Hayreh SS. Acute retinal arterial occlusive disorders. Prog Retin Eye Res. 2011; 30(5):359–394. PMID:
21620994.

2. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009; 116(10):1928–1936. PMID:
19577305.
3. Wilson LA, Warlow CP, Russell RW. Cardiovascular disease in patients with retinal arterial occlusion. Lancet. 1979; 1(8111):292–294. PMID:
84946.

4. Chang YS, Jan RL, Weng SF, Wang JJ, Chio CC, Wei FT, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012; 154(4):645–652.e1. PMID:
22809785.

5. Klein R, Klein BE, Jensen SC, Moss SE, Meuer SM. Retinal emboli and stroke: the beaver dam eye study. Arch Ophthalmol. 1999; 117(8):1063–1068. PMID:
10448750.
6. Park SJ, Choi NK, Yang BR, Park KH, Lee J, Jung SY, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015; 122(11):2336–2343.e2. PMID:
26298716.
7. Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015; 122(9):1881–1888. PMID:
26231133.
8. Chang YS, Chu CC, Weng SF, Chang C, Wang JJ, Jan RL. The risk of acute coronary syndrome after retinal artery occlusion: a population-based cohort study. Br J Ophthalmol. 2015; 99(2):227–231. PMID:
25147366.

9. Youn TS, Lavin P, Patrylo M, Schindler J, Kirshner H, Greer DM, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol. 2018; 265(2):330–335. PMID:
29236169.

10. Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C. 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J. 2007; 28(24):3042–3050. PMID:
17981829.

11. Hulten EA, Carbonaro S, Petrillo SP, Mitchell JD, Villines TC. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011; 57(10):1237–1247. PMID:
21145688.
12. Bittencourt MS, Hulten E, Ghoshhajra B, O'Leary D, Christman MP, Montana P, et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging. 2014; 7(2):282–291. PMID:
24550435.

13. Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010; 56(22):1864–1894. PMID:
21087721.
14. Hur J, Choi BW. Cardiac CT imaging for ischemic stroke: current and evolving clinical applications. Radiology. 2017; 283(1):14–28. PMID:
28318443.

15. Lee H, Yoon YE, Park JB, Kim HL, Park HE, Lee SP, et al. The incremental prognostic value of cardiac computed tomography in comparison with single-photon emission computed tomography in patients with suspected coronary artery disease. PLoS One. 2016; 11(8):e0160188. PMID:
27486804.

16. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990; 15(4):827–832. PMID:
2407762.

17. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975; 51(4):Suppl. 5–40. PMID:
1116248.

18. Al-Mallah MH, Qureshi W, Lin FY, Achenbach S, Berman DS, Budoff MJ, et al. Does coronary CT angiography improve risk stratification over coronary calcium scoring in symptomatic patients with suspected coronary artery disease? Results from the prospective multicenter international CONFIRM registry. Eur Heart J Cardiovasc Imaging. 2014; 15(3):267–274. PMID:
23966421.

19. Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007; 50(12):1161–1170. PMID:
17868808.

20. Leber AW, Becker A, Knez A, von Ziegler F, Sirol M, Nikolaou K, et al. Accuracy of 64-slice computed tomography to classify and quantify plaque volumes in the proximal coronary system: a comparative study using intravascular ultrasound. J Am Coll Cardiol. 2006; 47(3):672–677. PMID:
16458154.
21. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018; 125(10):1597–1607. PMID:
29716787.
22. Calvet D, Touzé E, Varenne O, Sablayrolles JL, Weber S, Mas JL. Prevalence of asymptomatic coronary artery disease in ischemic stroke patients: the PRECORIS study. Circulation. 2010; 121(14):1623–1629. PMID:
20351236.
23. Amarenco P, Lavallée PC, Labreuche J, Ducrocq G, Juliard JM, Feldman L, et al. Prevalence of coronary atherosclerosis in patients with cerebral infarction. Stroke. 2011; 42(1):22–29. PMID:
21088246.

24. Hong JH, Sohn SI, Kwak J, Yoo J, Ahn SJ, Woo SJ, et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. PLoS One. 2017; 12(6):e0177663. PMID:
28570629.

25. Babikian V, Wijman CA, Koleini B, Malik SN, Goyal N, Matjucha IC. Retinal ischemia and embolism. Etiologies and outcomes based on a prospective study. Cerebrovasc Dis. 2001; 12(2):108–113. PMID:
11490104.
26. Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med. 2007; 167(11):1145–1151. PMID:
17563022.

27. Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004; 328(7455):1519. PMID:
15213107.

28. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998; 97(18):1837–1847. PMID:
9603539.

29. Crea F, Libby P. Acute coronary syndromes: the way forward from mechanisms to precision treatment. Circulation. 2017; 136(12):1155–1166. PMID:
28923905.
30. Hankey GJ, Slattery JM, Warlow CP. Prognosis and prognostic factors of retinal infarction: a prospective cohort study. BMJ. 1991; 302(6775):499–504. PMID:
2012845.

31. Leisser C. Are there differences between internal carotid artery and aortic arch plaques among patients with retinal artery occlusion and anterior ischaemic optic neuropathy? Klin Monatsbl Augenheilkd. 2014; 231(11):1084–1087. PMID:
25003627.
32. Callizo J, Feltgen N, Ammermann A, Ganser J, Bemme S, Bertelmann T, et al. Atrial fibrillation in retinal vascular occlusion disease and non-arteritic anterior ischemic optic neuropathy. PLoS One. 2017; 12(8):e0181766. PMID:
28771491.

33. Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008; 52(21):1724–1732. PMID:
19007693.
34. Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008; 359(22):2324–2336. PMID:
19038879.

35. Guaricci AI, Pontone G, Brunetti ND, De Rosa F, Montrone D, Guglielmo M, et al. The presence of remodeled and mixed atherosclerotic plaques at coronary CT angiography predicts major cardiac adverse events - The CAFÉ-PIE Study. Int J Cardiol. 2016; 215:325–331. PMID:
27128555.

36. Maffei E, Seitun S, Martini C, Aldrovandi A, Cervellin G, Tedeschi C, et al. Prognostic value of computed tomography coronary angiography in patients with chest pain of suspected cardiac origin. Radiol Med. 2011; 116(5):690–705. PMID:
21424322.

37. SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015; 385(9985):2383–2391. PMID:
25788230.
38. Maffei E, Midiri M, Russo V, Rengo M, Tedeschi C, Spagnolo P, et al. Rationale, design and methods of CTCA-PRORECAD (Computed Tomography Coronary Angiography Prognostic Registry for Coronary Artery Disease): a multicentre and multivendor registry. Radiol Med. 2013; 118(4):591–607. PMID:
23358817.

39. Cho I, Al'Aref SJ, Berger A, Ó Hartaigh B, Gransar H, Valenti V, et al. Prognostic value of coronary computed tomographic angiography findings in asymptomatic individuals: a 6-year follow-up from the prospective multicentre international CONFIRM study. Eur Heart J. 2018; 39(11):934–941. PMID:
29365193.

40. Auer J. Coronary evaluation in patients with stroke: recognizing the risk. Atherosclerosis. 2015; 238(2):427–429. PMID:
25528683.

41. Hur J, Lee KH, Hong SR, Suh YJ, Hong YJ, Lee HJ, et al. Prognostic value of coronary computed tomography angiography in stroke patients. Atherosclerosis. 2015; 238(2):271–277. PMID:
25544177.

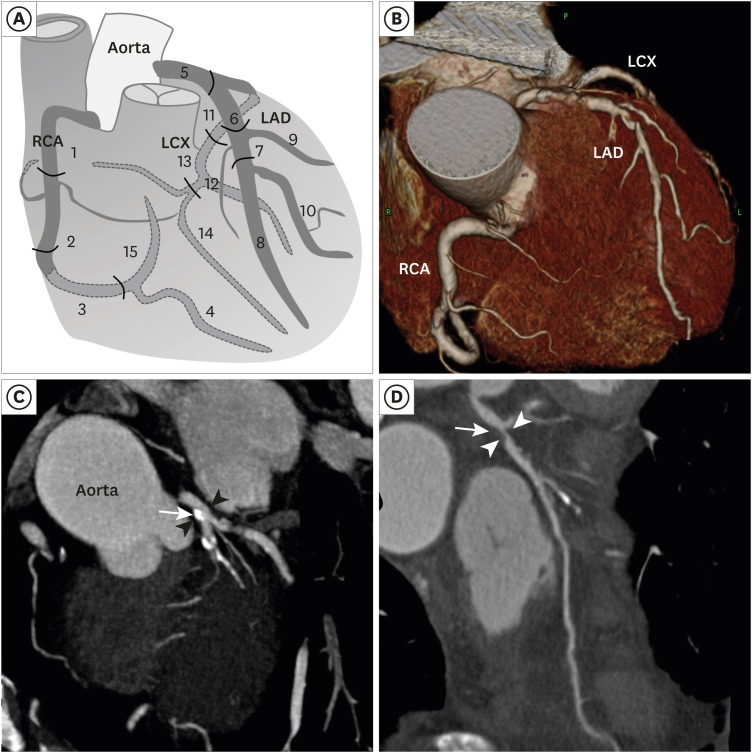
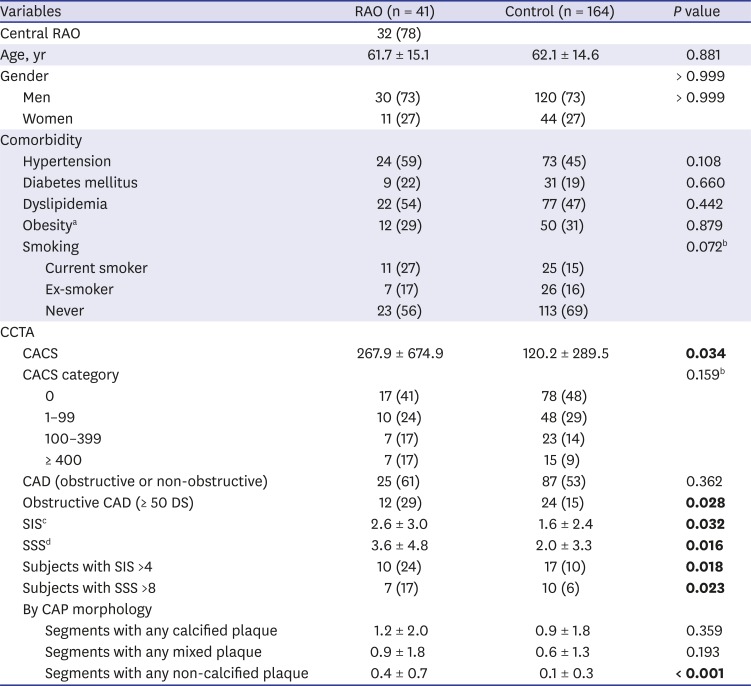
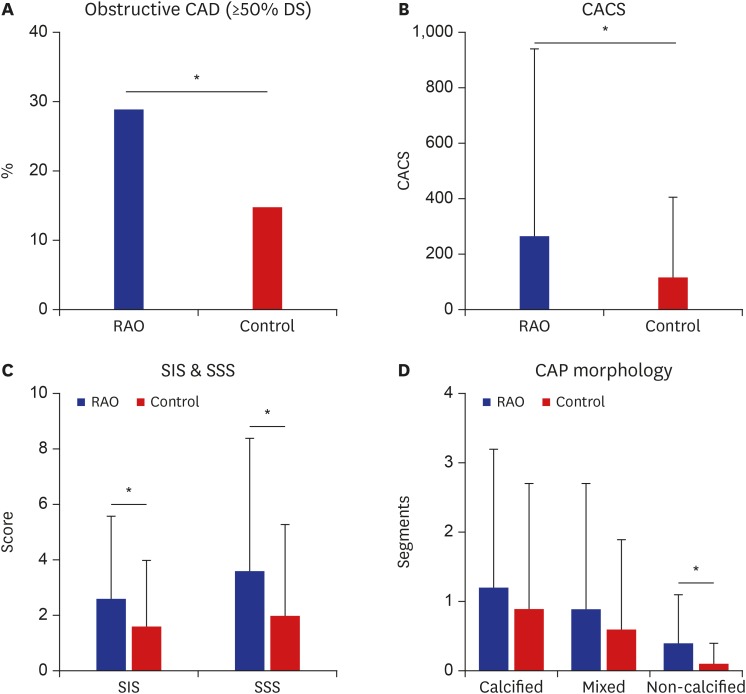
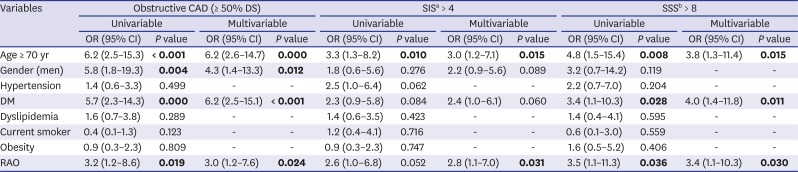




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download