Abstract
Purpose
Materials and Methods
Results
Figures and Tables
Fig. 1
The role of miR-222-3p in staphylococcal enterotoxin B (SEB)-mediated liver injury in mice. Wild type (WT) and miR-222 knockout (KO) mice were treated with 20 mg of D-galactosamine (D-gal) for 1 h, followed by 40 µg of SEB injection for 24 h or not. (A) Levels of miR-222 serum were confirmed by real-time quantitative PCR. Data were presented by 2−ΔΔCt value and normalized to WT control. (B and C) Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using special assay kits. (D) Histopathological examination of liver tissues was conducted using hematoxylin-eosin (HE) staining. Magnification: ×100. (E) Liver-infiltrating mononuclear cells were obtained by density gradient centrifugation, and total number of viable cells were counted using a hemocytometer. (F-I) Serum interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-2 were detected by enzyme-linked immunosorbent assay. *p<0.05. KO control group (without SEB challenge) versus WT control group (without SEB challenge); KO SEB (with standard error of mean challenge) group versus KO control group and WT SEB group, respectively; WT SEB group versus WT control group.

Fig. 2
Knockdown of miR-222-3p inhibited staphylococcal enterotoxin B (SEB)-induced inflammatory injury in splenocytes. Splenocytes from C57BL/6 mice were transfected with miR-222-3p/NC inhibitor (miR-222-3p/NC-in), and then challenged with 1 µg/mL of SEB for 24 h. (A) Expression levels of miR-222-3p were measured using real-time quantitative PCR. Data was presented by 2−ΔΔCt value and normalized to miR-NC-in. (B) Treated splenocytes were collected, and total cell number was determined by a hemocytometer. (C-F) Interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-2 in culture supernatant were detected by enzyme-linked immunosorbent assay. *p<0.05. miR-222-3p-in group (without SEB challenge) versus miR-NC-in group; SEB+miR-222-3p-in group versus miR-222-3p-in group and SEB+miR-NC-in group, respectively; SEB+miR-NC-in group versus miR-NC-in group.

Fig. 3
Suppressors of cytokine signaling 1 (SOCS1) was negatively regulated by miR-222-3p in splenocytes. (A and B) Real-time quantitative PCR (RT-qPCR) detected expression of SOCS1 in staphylococcal enterotoxin B (SEB)-induced mice and splenocytes. Data was presented by 2−ΔΔCt value and normalized to control. (C) The predicted miR-222-3p binding sites in mouse SOCS1 gene wild type (SOCS1-Wt) according to targetScan software. Corresponding sequence in the mutated version (SOCS1-Mut) was also shown. (D) Levels of miR-222-3p were confirmed in splenocytes when transfected with miR-222-3p mimic (miR-222-3p), inhibitor, or its corresponding control. (E and F) Luciferase activity of SOCS1 wild type (SOCS1-Wt) or SOCS1-Mut in splenocyte cells transfected with miR-222-3p/NC mimic (miR-222-3p/NC) or miR-222-3p/NC-in. (G and H) Expression levels of SOCS1 were confirmed by RT-qPCR and western blot in splenocytes when transfected with miR-222-3p, miR-222-3p-in, or its corresponding control. *p<0.05 compared to controls. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
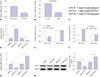
Fig. 4
Overexpression of suppressors of cytokine signaling 1 (SOCS1) suppressed inflammatory cytokines release in staphylococcal enterotoxin B (SEB)-induced splenocytes ex-vivo. Splenocytes from C57BL/6 mice were transfected with pcDNA-SOCS1/Vector (SOCS1/Vector), and then challenged with 1 µg/mL of SEB for 24 h. (A) Expression of SOCS1 was measured by western blot. GAPDH expression was used as internal reference. (B) Treated splenocytes were collected, and total cell number was determined by a hemocytometer. (C–F) Interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-2 in culture supernatant were detected by enzyme-linked immunosorbent assay. *p<0.05. SOCS1 group (without SEB challenge) versus Vector group; SEB+SOCS1 group versus SOCS1 group and SEB+Vector group, respectively; SEB+ SOCS1 group versus Vector group.

Fig. 5
Suppressors of cytokine signaling 1 (SOCS1) downregulation blocked the suppressive effect of miR-222-3p knockdown in staphylococcal enterotoxin B (SEB)-induced splenocytes. Splenocytes were transfected with miR-222-3p/NC, or co-transfected with miR-222-3p-in and si-SOCS1/NC. (A) Western blot showed SOCS1 levels after transfection. GAPDH level was used as internal reference. (B) Transfected splenocytes were collected, and total cell number was determined by a hemocytometer. (C-F) Interferon-gamma (INF-γ), tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and IL-2 in culture supernatant were detected by enzyme-linked immunosorbent assay. *p<0.05 compared to miR-NC-in group or miR-222-3p+si-NC group.

Notes
AUTHOR CONTRIBUTIONS
Conceptualization: Yifang Gui.
Methodology: Jingda Yu and Cui Sun.
Formal analysis: Peng Zhang and Weiping Han.
Data curation: Jingda Yu and Weiping Han.
Software: Weiping Han.
Validation: Jingda Yu.
Investigation: Cui Sun and Peng Zhang.
Writing—original draftt preparation: Peng Zhang and Jingda Yu.
Writing—review and editing: Peng Zhang, Jingda Yu, and Yifang Gui.
Approval of final manuscript: All authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download