Abstract
Purpose
To investigate if inflammation as a potential cause of false-positive lesions from recent UroNav magnetic resonance imaging (MRI) fusion prostate biopsy patients.
Materials and Methods
We retrospectively identified 43 men with 61 MRI lesions noted on prostate MRI before MRI ultrasound-guided fusion prostate biopsy. Men underwent MRI with 3T Siemens TIM Trio MRI system (Siemens AG, Germany), and lesions were identified and marked in DynaCAD system (Invivo Corporation, USA) with subsequent biopsy with MRI fusion with UroNav. We obtained targeted and standard 12-core needle biopsies. We retrospectively reviewed pathology reports for inflammation.
Results
We noted a total of 43 (70.5%) false-positive lesions with 28 having no cancer on any cores, and 15 lesions with cancer noted on systematic biopsy but not in the target region. Of the men with cancer, 6 of the false positive lesions had inflammation in the location of the targeted region of interest (40.0%, 6/15). However, when we examine the 21/28 lesions with an identified lesion on MRI with no cancer in all cores, 54.5% had inflammation on prostate biopsy pathology (12/22, p=0.024). We noted the highest proportion of inflammation.
Prostate cancer is the most common non-cutaneous cancer in American men [1]. Since the implementation of prostate-specific antigen screening in the 1990s, urologists perform on men a non-targeted, template prostate needle biopsy in order to diagnose prostate cancer [2]. Standard template biopsy suffers from sampling error noted by the 30% risk of upgrading at the time of prostatectomy and considering that only 30% to 40% of men who undergo the procedure are diagnosed with prostate cancer [3].
Prostate magnetic resonance imaging (MRI) is an imaging modality that may allow more accurate prostate biopsies. Advances in MRI technology have also led to techniques allowing fusion of the MRI images onto the standard ultrasound (US) equipment [4]. Armed with the tools to direct the biopsy to a particular area, urologists have expected improved detection of more aggressive tumors and potentially reduced the number of biopsies with negative MRIs. However, in the recent article by the PRECISION (Prostate Evaluation for Clinically Important Disease: Sampling Using Image Guidance or Not?) group, randomized men obtained a prostate biopsy based on MRI findings compared to a standard approach without MRI [5]. MRI only improved the detection rate of clinically significant cancer by 12% (95% confidence interval, 4 to 20; e.g., from 26% to 38%). While the result was statistically positive, we argue that a 38% detection rate is still quite weak. We consider other solid organ biopsies which typically reach a detection rate of more than 90% [6]. While MRI does provide incremental benefits to improve cancer detection, in our practice, we have noted a high false-positive rate that could be influencing the accuracy of prostate MRI.
Inflammation is known to mimic prostate cancer lesions on MRI, for example, chronic prostatitis or nodules following bacillus Calmette–Guérin treatment [789]. However, it is unknown how commonly inflammation plays a role in MRI fusion ultrasound-guided (MRI-US) prostate needle biopsies. We investigate inflammation identified on pathology reports from recent UroNav MRI fusion prostate biopsy patients.
After local IRB approval (approval number: HSC21000480H, University of Texas Health Science Center San Antonio [UTHSCSA]), we retrospectively identified 43 men with 61 MRI lesions noted on prostate MRI before MRI-US fusion prostate biopsy. The patients were consecutive and the only patients in at the UTHSCSA who had a fusion biopsy. There was no specific inclusion or exclusion criteria other than needing to have a fusion biopsy.
The MRI's were performed at our local institution, including two different scanners (UT Health: 3T Siemens TIM Trio MRI system [Siemens AG, Erlangen, Germany] and University Hospital: Phillips 3T [Andover, MA, USA]), with a pelvic phased-array coil, with or without an endorectal coil. After MRI, a body MRI radiologist would identify target lesions and outline the region of interest with the DynaCAD system (Invivo Corporation, Gainesville, FL, USA). We acquired T2-weighted, diffusion-weighted imaging, and dynamic contrast-enhanced sequences with B values of (50, 400, 800, 1,400). Our radiologists categorized lesions according to the Prostate Imaging–Reporting and Data System, version 2 (PI-RADS 2) with a score from one to five [10]. All MRI's were imported into the DynaCAD system by one radiologist for standard (A.S.) and reviewed by a urologist before biopsy (M.A.L.).
All patients underwent an enema before the procedure and antibiotics for less than 24 hours starting the morning of the procedure. We performed MRI fusion standard techniques using the UroNav fusion biopsy system. A single surgeon (M.A.L.) performed biopsies at the same location in a surgery center with sedation as needed. We performed standard scanning and segmentation with alignment before prostate biopsy attempt. We performed the biopsy of targeted lesions before a standard 12-core needle biopsy. A target lesion was biopsied three times (two sagittal and one transverse view). If only one lesion were present and if there were more than one lesion, we took two cores of each lesion. We utilized the UroNav systematic core biopsy guided system to record cores, and we did not attempt to exclude previously targeted core regions.
Samples were sent to pathology in formalin as the standard-of-care biopsies. We sent each core in a separate container with labels as the target and each systematic core. We retrospectively reviewed pathology reports for inflammation. After a review of selected cases, a representative case was utilized to display focal inflammation-mimicking cancer.
We identified a total of 43 patients who underwent MRI fusion biopsy for which we biopsied 61 targeted lesions. We identified cancer on 22 (51.2%) of the biopsies. We display demographics comparing false-positive lesions (no cancer) to true-positive lesions (cancer) in Table 1. The prostate specific antigen (PSA) density was significantly lower in the false-positive group than the those diagnosed with cancer (median, 0.08 vs. 0.14; p=0.02). Men who have had a previous biopsy were more likely to have a false-positive MRI reading (90.5% vs. 63.6%, p=0.04). Pathologic inflammation was identified in the pathology reports and all specified chronic inflammation. We then compared those with any inflammation (n=18) compared to those without inflammation (n=25). Age, body mass index, prostate size, PSA, white blood cells (WBCs) on urine analysis, race, smoking status, previous prostate biopsy, and use of finasteride were all non-significant (p>0.05). However, PSA density was actually lower in those with any inflammation (mean, 0.11; median [interquartile range, IQR], 0.11 [0.05–0.13]) compared to those without documented inflammation on their biopsy (mean, 0.22; median [IQR], 0.14 [0.9–0.31], p=0.02).
Of the 61 targeted lesions, 28 (45.9%) did not identify as cancer on any cores from the biopsy, including both the systematic and target lesions for that prostate. The most accurate definition of a false positive is a lesion that was a recognized lesion on MRI, but both biopsy and systematic cores were negative for cancer (false positive biopsy [FP]−, e.g., all cores negative for cancer). Our second definition of a false-positive lesion includes any MRI-detected lesion in which we identified no cancer (negative) on targeted biopsy specifically; however, would include cancer in the systematic cores (FP+, e.g., target negative with and without cancer detection on systematic biopsy). There were four (4/28, 14.3%) lesions negative for cancer yet had positive core biopsy on the systematic biopsy in the region of the target (one PI-RADS 3, and three PI-RADS 4). The targeted biopsies may have missed the lesions; however, due to the subtlety in determining if we genuinely missed the target or not, we included them in the false-positive category for purposes of analysis as this is a difficult determination.
This group of men has entirely negative prostate biopsies and could have potentially avoided biopsy. We display the locations of these false-positive lesions in Fig. 1. In Table 1, we labeled these men as the 21 subjects [48.8% (21/43)] with an actual false-positive targeted biopsy and showed no demographic differences. Inflammation was found in the target lesion in 47.6% (10/21) of lesions identified on MRI in this group.
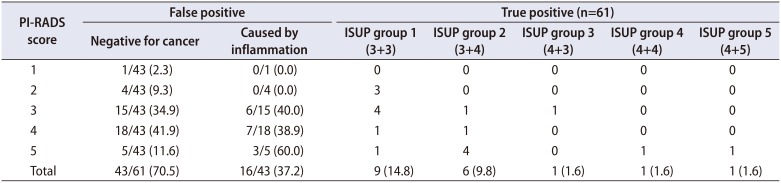
We display the PI-RADS score for each lesion in Table 2. Seven lesions did not have PI-RADS scores and needed re-evaluation, as scans had come from outside facilities in South Texas. In the true-positive lesions, 9/18 (50.0%) were clinically significant cancers (Gleason >3+4, International Society of Urological Pathology [ISUP] group >2). The proportion of clinically significant cancers is higher in those PI-RADS scores >3 (77.8% [7/9] vs. 22.2% [2/9], p=0.02). Taking all 61 lesions into account, 70.5% (43/61) had a false-positive targeted lesion (FP+). More than 50% of the FP+ lesions were PI-RADS >3 (23/43, 53.5%), of which inflammation caused nearly half (16/43 [37.2%] in FP+). We labeled the locations of these lesions in Fig. 2. Only three (7.0%, 3/43) patients had the target lesion alone be the only site of cancer detection. Moreover, the targeted lesion did not provide a higher Gleason grade than the systematic cores in any patient.
Inflammation was not mentioned in the pathology report (0/33) if any cancer was present on the biopsy. Therefore, inflammation was not mentioned in the presence of cancer and could be underreported in the cancerous prostate, even if the target lesions may have had inflammation. As an example of one of the false-positive lesions caused by inflammation, we provided a summary figure to display the image and pathology (Fig. 3). We show the MRI images, Uronav MRI-US fusion system and pathologic images at 4×, 10×, and 20× magnification. Of the 22 cancerous lesions, 12 (54.5%) were Gleason 3+3 (grade group 1).
We identified that our false-positive rate for patients presented with an MRI targetable lesion was 70.5% (43/61). Despite popular press and improved targeting of lesions, it is evident that improved imaging techniques to reduce false-positive rates are urgently needed. The American Urological Association and the Society of Abdominal Radiology released a joint consensus statement to clarify the utilization of prostate MRI in patients with previous negative biopsies or men on active surveillance (AS). A recent publication from the ASIST trial regarding MRI in patients on AS showed targeted biopsy did not improve upgrading, and systematic biopsies were still necessary [11]. In another context, the use of MRI before the initial biopsy is gaining in popularity [12]. Unfortunately, false-positive lesions continue to plague the accuracy of prostate MRI. Jyoti and colleagues published their experience of in-gantry MRI biopsies from Australia (n=137) noting that PI-RADS 3 and 4 lesions with inflammation accounted for 97% of the false-positive lesions mainly in the transition zone (54%) [9].
Chronic prostatitis shows variable signal intensity and even mimics cancer using MR-spectroscopy with choline peeks and limited citrate values [13]. Nagel et al. [14] identified significant differences in the value called median apparent diffusion coefficients (ADC) between normal prostate, prostatitis, low- and high-grade cancers (p<0.001). However, the report noted that the ADC values overlapped between the groups and are not likely to be clinically useful. A novel MRI acquisition protocol named restriction spectrum imaging (RSI) may be able to parse the signal of inflammation and cancer [15]. RSI-MRI in the prostate has specifically been shown to be more accurate than ADC values, improve tumor conspicuity, and alter signal between inflammation and cancer [16171819]. Other groups have investigated more efficient use of intravenous contrast enhancement techniques to distinguish aggressive tumors [20]. Several other computational measures have been explored to improve tumor conspicuity [21].
As the use of MRI utilization increases for lower risk individuals, such as for prostate cancer screening, we would like to highlight the possibility of inflammation as a potential source for false positive findings. Future studies would ideally focus on improving acquisition protocols to reduce noise from inflammation and potentially combine with prostate inflammation biomarkers. Biomarkers may provide inside into the amount of focal inflammation and allow readers to temper suspicion levels or reduce inflammation and repeat the scan. We did not demonstrate the urinary WBC count on urine analysis to be useful, though other studies have demonstrated this possibility [22]. Despite some interest in the prostate cancer antigen gene 3 (PCA3) urine-based biomarker to determine subsequent MRI and targeted prostate biopsy, studies have shown that PCA3 and other biomarkers may not distinguish between prostatitis and prostate cancer [23]. One study identified SelectMDx biomarker (MDxHealth, Irvine, CA, USA) may be superior to PCA3 in this context [24].
The MRI fusion prostate biopsy is not without its limitations. There is a significant learning curve for the team over time, which included urologists, pathologists, radiologists and supporting staff [25]. Our data for this study do include our initial biopsy experience and may include missed targeted lesions. Guidelines continue to recommend performing the systematic biopsy along with the targeted approach because an additional 15% of cancers are identified [26]. We retrospectively reviewed our pathology reports, as we did not have adequate funding for the pathology to re-read all previous cores. Our sample size is small and will need larger, prospective targeted studies on this topic to make more definitive statements regarding inflammation and its appearance on multiparametric MRI (mpMRI). We have a low rate of clinically significant prostate cancer detection and is likely due to our population of men with large prostates and negative biopsy and men on AS. When cancer is present, identification of specific inflammation may be minimized, thus reported less. Non-uniformity in the MRI reading of prostate lesions likely leads to more variation and less confidence regarding particular inflammatory lesions. Therefore, artificial intelligence with deep learning of images, among other enhancement processes, may provide more accurate alerts to assist radiologists with false-positive MRI lesions.
ACKNOWLEDGMENTS
This work was supported by the NIH (P30CA054174) the Office of the Assistant Secretary of Defense for Health Affairs through the Prostate Cancer Research Program under Award No. W81XWH-15-1-0441. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; 67:7–30. PMID: 28055103.

2. Haffty BG, Lawton CA, Sandler H. Watchful waiting-active surveillance in low-risk prostate cancer. JAMA Oncol. 2015; 1:688–689.

3. Filippou P, Welty CJ, Cowan JE, Perez N, Shinohara K, Carroll PR. Immediate versus delayed radical prostatectomy: updated outcomes following active surveillance of prostate cancer. Eur Urol. 2015; 68:458–463. PMID: 26138041.

4. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015; 313:390–397. PMID: 25626035.

5. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. PRECISION Study Group Collaborators. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018; 378:1767–1777. PMID: 29552975.

6. Marconi L, Dabestani S, Lam TB, Hofmann F, Stewart F, Norrie J, et al. Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol. 2016; 69:660–673. PMID: 26323946.
7. Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Lisi D, Osimani M, et al. Magnetic resonance spectroscopic imaging (1H-MRSI) and dynamic contrast-enhanced magnetic resonance (DCE-MRI): pattern changes from inflammation to prostate cancer. Cancer Invest. 2010; 28:424–432. PMID: 20073578.

8. Cheng Y, Zhang X, Ji Q, Shen W. Xanthogranulomatous prostatitis: multiparametric MRI appearances. Clin Imaging. 2014; 38:755–757. PMID: 24852675.

9. Jyoti R, Jina NH, Haxhimolla HZ. In-gantry MRI guided prostate biopsy diagnosis of prostatitis and its relationship with PIRADS V.2 based score. J Med Imaging Radiat Oncol. 2017; 61:212–215. PMID: 27987276.

10. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging-reporting and data system: 2015, version 2. Eur Urol. 2016; 69:16–40. PMID: 26427566.
11. Klotz L, Loblaw A, Sugar L, Moussa M, Berman DM, Van der Kwast T, et al. Active Surveillance Magnetic Resonance Imaging Study (ASIST): results of a randomized multicenter prospective trial. Eur Urol. 2019; 75:300–309. PMID: 30017404.

12. Rosenkrantz AB, Verma S, Choyke P, Eberhardt SC, Eggener SE, Gaitonde K, et al. Prostate magnetic resonance imaging and magnetic resonance imaging targeted biopsy in patients with a prior negative biopsy: a consensus statement by AUA and SAR. J Urol. 2016; 196:1613–1618. PMID: 27320841.

13. Shukla-Dave A, Hricak H, Eberhardt SC, Olgac S, Muruganandham M, Scardino PT, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings--initial observations. Radiology. 2004; 231:717–724. PMID: 15163811.
14. Nagel KN, Schouten MG, Hambrock T, Litjens GJ, Hoeks CM, ten Haken B, et al. Differentiation of prostatitis and prostate cancer by using diffusion-weighted MR imaging and MR-guided biopsy at 3 T. Radiology. 2013; 267:164–172. PMID: 23329653.

15. White NS, McDonald C, Farid N, Kuperman J, Karow D, Schenker-Ahmed NM, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014; 74:4638–4652. PMID: 25183788.

16. Liss MA, White NS, Parsons JK, Schenker-Ahmed NM, Rakow-Penner R, Kuperman JM, et al. MRI-derived restriction spectrum imaging cellularity index is associated with high grade prostate cancer on radical prostatectomy specimens. Front Oncol. 2015; 5:30. PMID: 25741473.

17. White NS, Leergaard TB, D'Arceuil H, Bjaalie JG, Dale AM. Probing tissue microstructure with restriction spectrum imaging: histological and theoretical validation. Hum Brain Mapp. 2013; 34:327–346. PMID: 23169482.

18. White NS, McDonald CR, Farid N, Kuperman JM, Kesari S, Dale AM. Improved conspicuity and delineation of high-grade primary and metastatic brain tumors using “restriction spectrum imaging”: quantitative comparison with high B-value DWI and ADC. AJNR Am J Neuroradiol. 2013; 34:958–964. S1PMID: 23139079.

19. Yamin G, Schenker-Ahmed NM, Shabaik A, Adams D, Bartsch H, Kuperman J, et al. Voxel level radiologic-pathologic validation of restriction spectrum imaging cellularity index with gleason grade in prostate cancer. Clin Cancer Res. 2016; 22:2668–2674. PMID: 27250935.

20. Othman AE, Falkner F, Martirosian P, Schraml C, Schwentner C, Nickel D, et al. Optimized fast dynamic contrast-enhanced magnetic resonance imaging of the prostate: effect of sampling duration on pharmacokinetic parameters. Invest Radiol. 2016; 51:106–112. PMID: 26447494.
21. Mayer R, Simone CB 2nd, Skinner W, Turkbey B, Choykey P. Pilot study for supervised target detection applied to spatially registered multiparametric MRI in order to non-invasively score prostate cancer. Comput Biol Med. 2018; 94:65–73. PMID: 29407999.

22. Tekin A, Yuksel A, Tekin S, Gumrukcu G, Aslan AR, Sengor F. Post-prostatic massage examination for prediction of asymptomatic prostatitis in needle biopsies: a prospective study. J Urol. 2009; 182:564–568. discussion 568–9. PMID: 19524953.

23. De Luca S, Passera R, Fiori C, Bollito E, Cappia S, Mario Scarpa R, et al. Prostate health index and prostate cancer gene 3 score but not percent-free prostate specific antigen have a predictive role in differentiating histological prostatitis from PCa and other nonneoplastic lesions (BPH and HG-PIN) at repeat biopsy. Urol Oncol. 2015; 33:424.e17–424.e23.

24. Hendriks RJ, van der Leest MMG, Dijkstra S, Barentsz JO, Van Criekinge W, Hulsbergen-van de Kaa CA, et al. A urinary biomarker-based risk score correlates with multiparametric MRI for prostate cancer detection. Prostate. 2017; 77:1401–1407. PMID: 28853167.

25. Meng X, Rosenkrantz AB, Huang R, Deng FM, Wysock JS, Bjurlin MA, et al. The institutional learning curve of magnetic resonance imaging-ultrasound fusion targeted prostate biopsy: temporal improvements in cancer detection in 4 years. J Urol. 2018; 200:1022–1029. PMID: 29886090.

26. Ploussard G, Borgmann H, Briganti A, de Visschere P, Fütterer JJ, Gandaglia G, et al. EAU-YAU Prostate Cancer Working Group. Positive pre-biopsy MRI: are systematic biopsies still useful in addition to targeted biopsies? World J Urol. 2019; 37:243–251. PMID: 29967944.

Fig. 1
(A) Proportion of false positives (no cancer identified) based on location. (B) Proportion of inflammation of false positives (FP−, no cancer identified) based on prostate biopsy location. R, right; L, left.
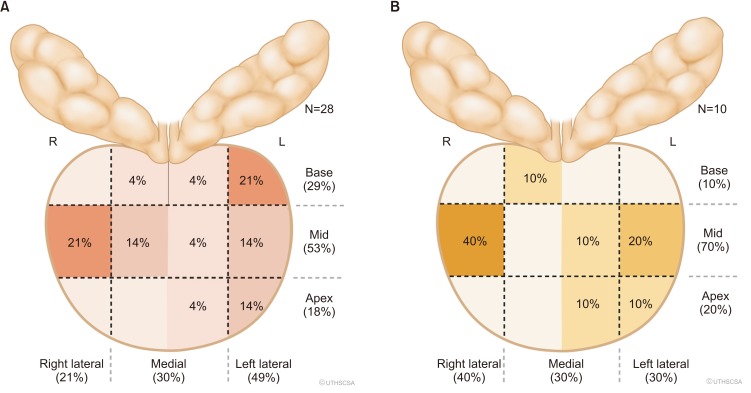
Fig. 2
Proportion of false positives (FP+, negative target with or without cancer on systematic prostate biopsy) based on prostate biopsy location. R, right; L, left.
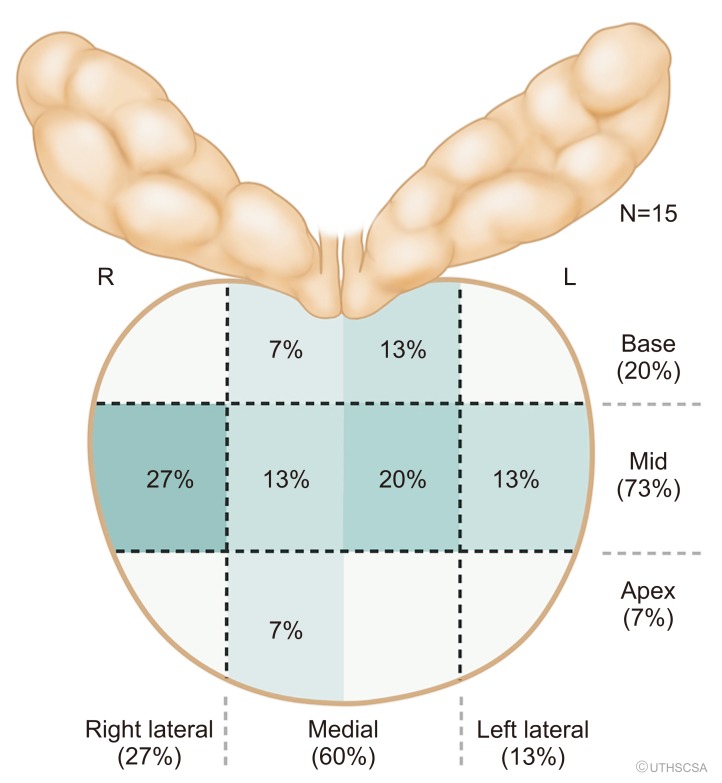
Fig. 3
Example of true-positive lesions and false positive caused by inflammation. We present the course of action of a 67-year-old man who presented with elevated prostate specific antigen (PSA) and a previous biopsy with high-grade (HG) PIN in one core of a 12-core biopsy. His magnetic resonance imaging (MRI), which originally was read as Prostate Imaging–Reporting and Data System (PI-RADS) 4, was downgraded to a PI-RADS 3 on re-review. He underwent MRI fusion with UroNav, and the biopsy is displayed. Only inflammation was noted in the pathology specimens displayed on the right side (hematoxylin and eosin stain, top down magnification is 4×, 10×, and 20×). DWI, diffusion-weighted imaging; DCE, dynamic contrast-enhanced.
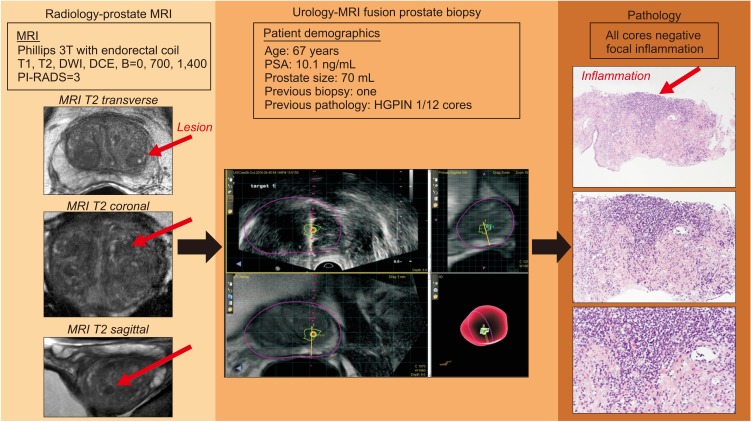
Table 1
Cohort demographics comparing false negative MRI lesions to true positive lesions on MRI ultrasound guided fusion prostate biopsy
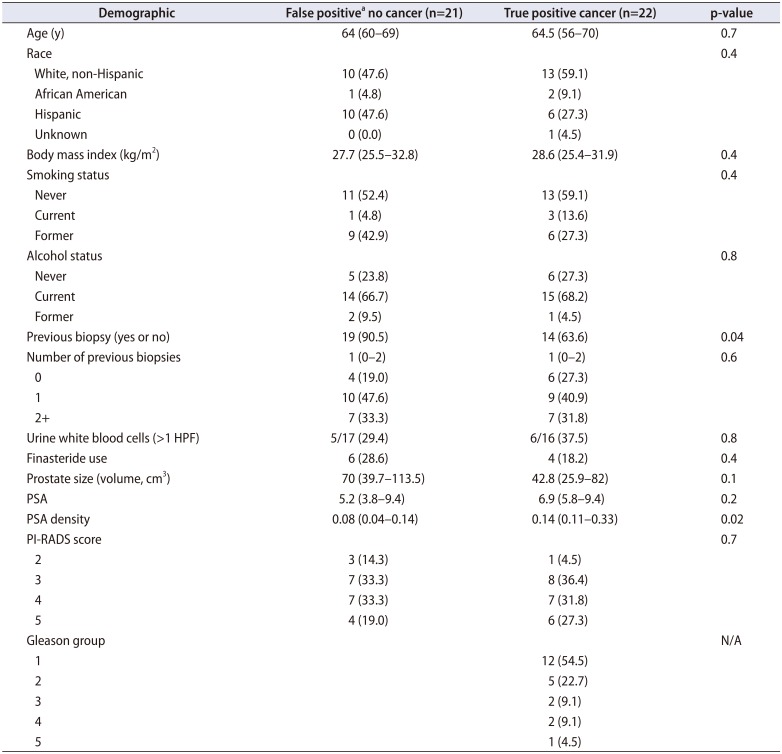
Values are presented as mean (range) or number (%).
MRI, magnetic resonance imaging; HPF, high-power field; PSA, prostate specific antigen; PI-RADS, Prostate Imaging–Reporting and Data System; N/A, not applicable.
a:False positive is defined that there was a lesion identified on MRI and no biopsies were identified with cancer.
Table 2
MRI identified lesions and associated PI-RADS score and pathology




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download