Abstract
Purpose
Materials and Methods
Results
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTIONS:
Conceptualization: Chi Young Shim.
Data curation: Soo Youn Lee and Hyeonju Jeong.
Formal analysis: Hyeonju Jeong and Chi Young Shim.
Investigation: Hyeonju Jeong.
Methodology: Hyeonju Jeong and Chi Young Shim.
Project administration: Chi Young Shim.
Resources: Hyeonju Jeong, Chi Young Shim, Darae Kim, Jah Yeon Choi, Kang-Un Choi, Soo Youn Lee, Geu-Ru Hong, and Jong-Won Ha.
Software: Hyeonju Jeong and Chi Young Shim.
References
Fig. 1
Prevalence of specific cardiomyopathies in bicuspid aortic valves (BAV) subjects. (A) A total of 1186 BAV subjects. (B) Sixty-seven BAV subjects with cardiomyopathy (CM). LVNC, left ventricular non-compaction; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy.
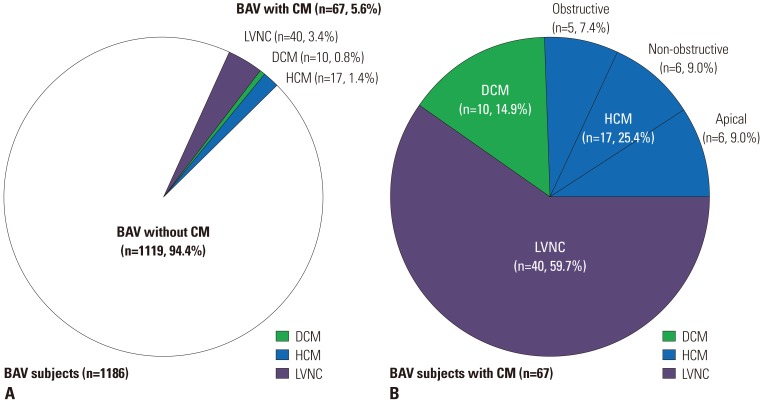
Fig. 2
(A) Bicuspid aortic valves (BAV) phenotypes according to the presence of specific cardiomyopathies (CMs). (B) Aorta phenotypes according to the presence of specific CMs. DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular non-compaction.
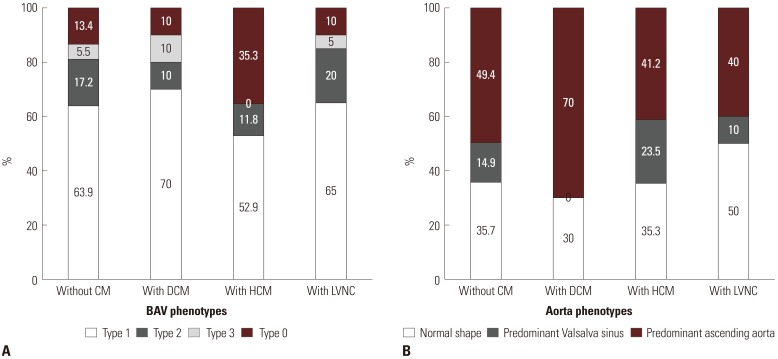
Fig. 3
Representative cases of coexisting cardiomyopathies in bicuspid aortic valves subjects. (A) Hypertrophic cardiomyopathy, (B) left ventricular non-compaction, and (C) dilated cardiomyopathy.
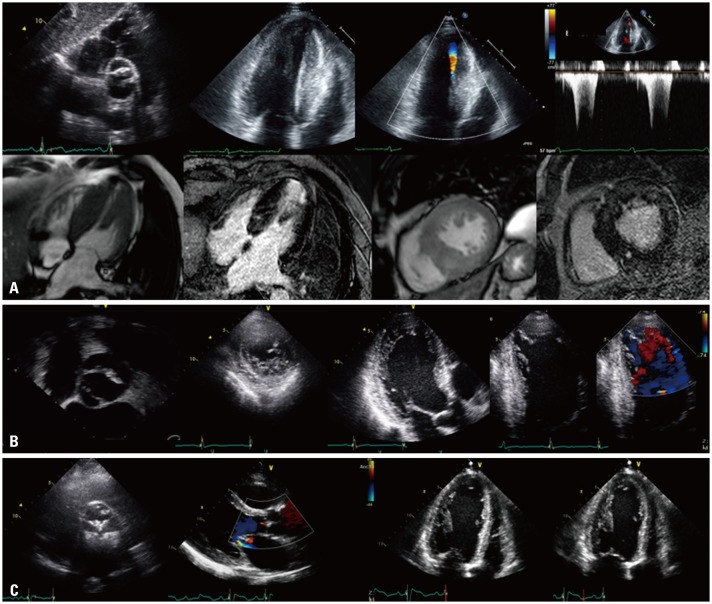
Table 1
Baseline Characteristics according to the Presence of Specific Cardiomyopathy
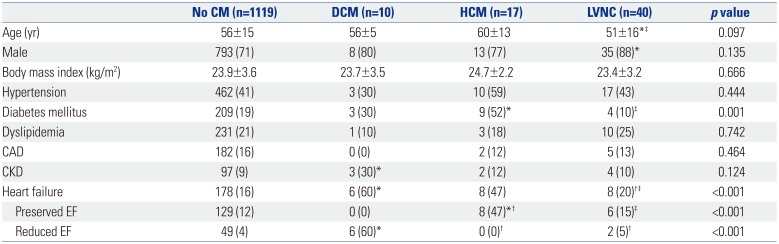
CM, cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular non-compaction; CAD, coronary artery disease; CKD, chronic kidney disease; EF, ejection fraction.
Values are presented as mean±standard deviation or n (%) unless otherwise indicated.
*p<0.05 compared to the group with no CM; †p<0.05 compared to the DCM group; ‡p<0.05 compared to the HCM group.
Table 2
Left Ventricle Characteristics according to the Presence of Specific Cardiomyopathy
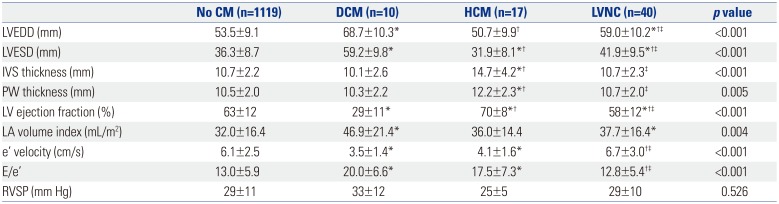
CM, cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular non-compaction; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; IVS, interventricular septum; PW, posterior wall; LV, left ventricle; LA, left atrium; e', early diastolic mitral annular tissue; E/e', ratio of early diastolic mitral inflow velocity to early diastolic mitral annular tissue velocity; RVSP, right ventricular systolic pressure.
Values are presented as mean±standard deviation unless otherwise indicated.
*p<0.05 compared to the group with no CM; †p<0.05 compared to the DCM group; ‡p<0.05 compared to the HCM group.
Table 3
BAV and Aorta Characteristics according to the Presence of Specific Cardiomyopathy
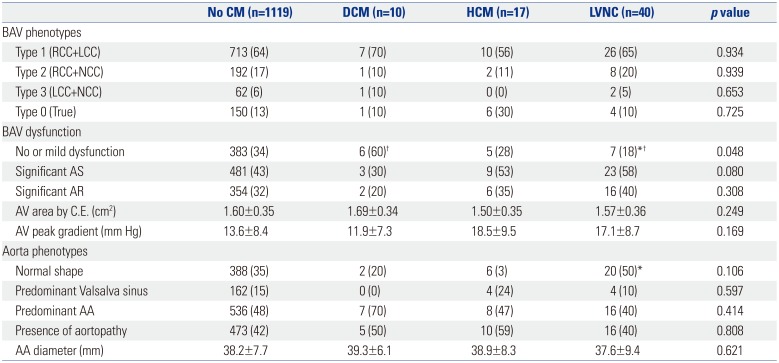
BAV, bicuspid aortic valve; CM, cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; LVNC, left ventricular noncompaction; RCC, right coronary cusp; LCC, left coronary cusp; NCC, non-coronary cusp; AS, aortic stenosis; AR, aortic regurgitation; AV, aortic valve; C.E., continuity equation; AA, ascending aorta.
Values are presented as mean±standard deviation or n (%) unless otherwise indicated.
*p<0.05 compared to the group with no CM; †p<0.05 compared to the DCM group.
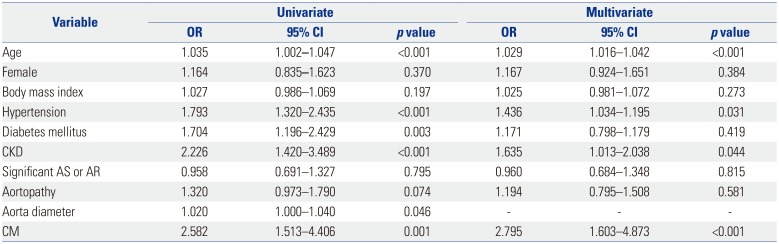
Table 4
Factors associated with Heart Failure in BAV Subjects




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download