Abstract
Managing acute pancreatitis is clinically challenging because of the generally poor patient condition, the variety of treatment options depending on the severity and complications, and the uncertainty of outcomes. Recently, the step-up approach, which involves less invasive initial treatment and more invasive subsequent treatment, where necessary, has been proposed as the mainstay of managing pancreatitis. This paper presents a case of a 57-year-old man with severe acute pancreatitis, who developed an unexpected fistula in the rectum, which was treated successfully using the step-up approach. In managing this case, the authors faced clinical challenges in determining the infection of necrotic tissue in the early phase of the disease, the optimal timing and method of drainage, and the fistula closure or repair technique. Successful management of this case using the step-up approach validated current recommendations and suggests that it is a reasonable treatment strategy for pancreatic-colonic fistulas. This case also highlights the importance of an awareness that pancreatitis-associated complications can develop in an unexpected manner.
Severe acute pancreatitis is a critical condition caused by the development of pancreatic and peri-pancreatic necrosis, organ failure and the possibility of subsequent infection.123 The infection of necrotic tissue, which is a major cause of mortality, is difficult to detect in the early phase of the disease, but requires prompt removal.1234 In the past, aggressive surgery with debridement was the treatment of choice, but it resulted in high mortality.1 In recent years, however, management has tended to be more noninvasive. In this regard, the “step-up” approach has been proposed as the mainstay in the management of pancreatitis and its complications, involving less invasive modalities initially, and then progressing stepwise to more invasive options.34 Nevertheless, treatment is still a clinical challenge owing to the generally poor patient condition, various treatment options depending on the severity or complications, and the uncertain outcome.
This paper presents a case of severe acute pancreatitis with fistula formation in the rectum, an unexpected and unusual complication site. The present report documents the challenges in critical decision-making in this case and emphasizes the importance of a multidisciplinary step-up approach in the management of severe acute pancreatitis and its complications.
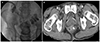
A 57-year-old man was admitted due to severe epigastric pain. The patient had been diagnosed with diabetes mellitus, hypertension, and alcoholic cirrhosis of the liver. Upon admission, his blood pressure, heart rate, and body temperature was 110/70 mmHg, 84 beats/min, and 38.0℃, respectively. The patient showed tenderness on the epigastrium. The laboratory examination revealed the following: white blood cells 7,290/µL (neutrophils, 76.9%); hemoglobin 11.2 g/dL; platelets 53×103/µL; AST 136 IU/L; ALT 21 IU/L; ALP 76 IU/L; GGT 376 IU/L; total bilirubin 2.30 mg/dL; amylase 1,776 IU/L; lipase 2,271 IU/L; BUN 43.5 mg/dL; creatinine 0.67 mg/dL; lactate dehydrogenase 835 IU/L; and CRP 68.18 mg/L. Abdominal CT revealed edema of the pancreatic head with slightly decreased enhancement. In addition, fluid collections were present with extension to the pelvic cavity, which was consistent with acute peripancreatic fluid collection (Fig. 1A, B). Intravenous (IV) fluid replacement was started as soon as the pancreatitis was detected on the laboratory and CT results. Normal saline and Hartmann's solution was supplied at a rate of 5 mL/kg/hr and then adjusted where necessary. Given the patient had fever at presentation, IV antibiotics were administered upon admission.
On day 3, his mental status became drowsy, and the patient showed hemodynamic instability (tachycardia 148 beats/min; blood pressure 69/35 mmHg) and he was transferred to the intensive care unit. His blood pH was 7.286, lactate level was elevated to 7.8 mmol/L, and partial pressure arterial oxygen was 78.5 mmHg with a nasal oxygen supply for 5 L/min. His white blood cell count was 12,630/µL (neutrophil, 75%), hemoglobin 9.5 g/dL. His renal function deteriorated with BUN and creatinine levels of 92.5 and 1.37 mg/dL, respectively. A blood culture at the time of admission showed no microorganisms. Mechanical ventilation was started given his drowsy mental status and unstable respiration. Although fluid replacement was continued, the hypotension persisted and an IV inotropic was needed for blood pressure control. At this point, continuing supportive care with IV antibiotics versus immediate drainage was discussed. For the management of pancreatitis, delaying drainage until complete walling-off of the lesion, which takes an average of four weeks after onset, is a widely accepted strategy. In addition, continue supportive treatment was decided because most pancreatic infections occur after the second week and that a white blood count elevation with organ failure can occur in sterile pancreatitis.45 On hospital day five, the hemodynamic instability improved and the IV inotropic was discontinued. Ventilator weaning was also possible on day 5 and extubation was performed on day 6. In the revised Atlanta classification, organ failure (two or more points in the modified Marshall score) for more than 48 hours is defined as severe acute pancreatitis and the current patient required an inotropic for blood pressure control for more than two days (two points in cardiovascular system: systolic blood pressure <90, not fluid responsive), resulting in a diagnosis of severe acute pancreatitis.6 The patient's condition became stable under supportive care and antibiotic therapy, and he was transferred back to the ward.
The patient showed clinical improvement, but on day 20, he suddenly developed a high fever (39.2℃) and abdominal pain with leukocytosis (14,200/µL) (neutrophils, 88%) and a CRP level of 169.5 mg/L. His general condition deteriorated, and a follow-up CT revealed massive peripancreatic and pelvic necrotic collections with wall formation (Fig. 1C). Although the antibiotic and fluid therapies were continued, his symptoms persisted, and an infection of the necrotic tissue was suspected. The need for drainage was inevitable but the optimal modality (endoscopic, percutaneous, or surgical) was uncertain. On CT, the necrotic collection revealed a deep extension into the pelvic cavity, making endoscopic drainage infeasible. Considering the patient's poor general condition and current trend of non-invasive management, a decision was made to perform percutaneous drainage, and drainage tubes were inserted into the pelvic cavity and retroperitoneum under fluoroscopy.
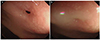
Although clinical improvement was noted after tube drainage, fecal matter was observed in the drainage tube on day 25, which was accompanied by an aggravation of abdominal pain. Sigmoidoscopy revealed a fistula opening in the rectum, 8 cm from the anal verge (Fig. 2). Radiography with contrast media injected via the drainage tube confirmed the communication between the walled-off necrosis and rectum (Fig. 3A). A follow-up CT also revealed a fistula tract (Fig. 3B). A diagnosis of pancreatic-rectal fistula was made, which is one of the most serious complications of pancreatitis and is associated with high mortality. Traditionally, pancreatic-colonic fistulas are treated surgically, but in recent years, there have been several reports on successful endoscopic closure of pancreatic- colonic fistulas.789 The patient's general condition was poor, and it was decided to perform endoscopic fistula closure to block further fecal contamination and maintain percutaneous drainage. Fistula closure with endoscopic clipping was performed, but the patient showed persistent signs of infection, abdominal pain, and drainage of fecal matter continued. The decision for surgery was then made, but a less invasive transient-diverting sigmoid colostomy was performed instead of a wide necrosectomy and fistula repair.
After colostomy formation, the tube drainage no longer showed fecal matter and the patient's condition stabilized. At 4 weeks after the colostomy, the patient was discharged from hospital with a prescription for oral antibiotics. A follow-up CT performed 2 months later revealed a decreased amount of necrotic tissue, and the percutaneous drainage tube was removed 4 months after the colostomy. Sigmoidoscopy also revealed complete closure of the fistula opening. Colostomy repair was then performed. The patient is currently undergoing outpatient follow-up with a good clinical course.
In recent years, the management of acute pancreatitis has changed in favor of a more noninvasive strategy.1234 In the past, the standard treatment for infected necrotizing pancreatitis was an open necrosectomy with complete removal of the infected necrotic tissue, which carries a high risk of complications. As a result, alternative techniques, such as minimally invasive surgery, percutaneous drainage, and endoscopic drainage, have been proposed and shown promising results.1234 Therefore, the concept of a “step-up” approach, designed and performed by a multidisciplinary team, has been proposed using recent positive multicenter randomized-control trial data.3 In this regard, the treatment guidelines for acute pancreatitis also recommend percutaneous or endoscopic drainage as an initial intervention for infected pancreatitis.4 On the other hand, data on the best drainage modality for specific patient subgroups are lacking and further investigations are needed. In the present case, the patient was treated based on the step-up approach, starting with antibiotics and then moving to percutaneous drainage.
Pancreatic-colonic fistula is a rare critical complication of acute pancreatitis, with a mortality rate of 17–67%.1011 The precise pathophysiological mechanism of pancreatic-enteric fistula formation is unknown, but several mechanisms or factors have been suggested, as reviewed by Shatney and Sosin.12 The combinations of the proposed factors are likely to be surgical in most cases. One proposed mechanism is the continuous secretion of pancreatic enzymes into the walled-off necrosis via communication with the pancreatic duct. This secretion might result in progressive digestion of the necrosis and its neighboring organs, which might in turn result in fistula formation. The increased intracystic pressure caused by fluid accumulation in the walled-off necrosis has also been suggested to compromise the weakest point of the wall and its vasculature, with subsequent necrosis and fistula formation. Other reported factors are the incidentally increased intra-abdominal pressure and abdominal trauma, such as needle aspiration or drainage. In the present case, the large size of the necrosis supports the idea of increased pressure. On the other hand, the possibility of iatrogenic fistula due to percutaneous drainage cannot be excluded. A pancreatic-enteric fistula usually arises near the pancreas, such as in the stomach, duodenum, or colon.13 Therefore, anatomical proximity might be an important factor related to the frequency with which an organ is involved. Thus, the splenic flexure and transverse colon are the most common sites in the colorectum.7 To the best of the authors' knowledge, this is the first report of pancreatic-colonic fistula formation in the rectum as a complication of acute pancreatitis. Given its distant location from the pancreas, the rectum is usually spared in colonic complications of pancreatitis. In the present case, the walled-off necrosis extended to the pelvic cavity near the rectum, producing an anatomical proximity.
In most cases of pancreatitis-associated upper gastrointestinal fistula formation, only supportive care is needed, and spontaneous closure is likely.1314 The fistula can even act as a good drainage route. In contrast, spontaneous closure is much less likely in colonic fistulas and they have a poor prognosis compared to fistula formation in other sites of the gastrointestinal tract.111314 Hence, pancreatic-colonic fistula is potentially a continuous infection source, rather than a drainage route, and prompt blocking of fecal contamination is crucial in its management. Traditionally, surgical debridement with fistula repair or colostomy formation has been performed, but there are several reports of successful endoscopic fistula closures by endoscopic clipping, endoloop, fibrin glue, and over-the-scope clipping.789 For non-severe cases, there have been case reports of successful treatment using only conservative measures, such as continuous lavage through a drainage tube or bowel cleansing alone.715 In the present patient, considering the potentially higher perioperative morbidity or mortality due to the patient's poor clinical status and the recently reported success of less invasive management, sigmoidoscopic fistula closure was performed and percutaneous drainage was maintained, but this approach was relatively ineffective. A more aggressive treatment option was considered and a simple transient diverting colostomy was performed to minimize the invasiveness of the operation. After the colostomy, the patient ultimately recovered without the need for further invasive surgery. This suggests that the step-up approach is a reasonable choice for pancreatic-colonic fistulas and supports the general utility of the approach in the management of pancreatitis and its complications.
This case of severe acute pancreatitis was complicated and severe, with unexpected rectal fistula formation. Although the patient's medical condition deteriorated and many clinical challenges arose, he was treated successfully based on the step-up approach. His recovery validates the value of this approach in the management of pancreatitis and suggests that it can be extended to the management of pancreatic-colonic fistulas. Nevertheless, physicians should be aware that necrotizing pancreatitis-associated complications can manifest in unexpected ways.
Figures and Tables
Fig. 1
Computed tomography (CT) scans of the patient. Initial CT scan reveals edematous change of the pancreatic head with acute peripancreatic fluid collection (A) extending to the pelvic cavity (B). Follow-up scan at day 20 shows a massive increase in necrotic collection and wall formation at the same level (C).

References
1. Zerem E. Treatment of severe acute pancreatitis and its complications. World J Gastroenterol. 2014; 20:13879–13892.

2. Zerem E, Imamović G, Sušić A, Haračić B. Step-up approach to infected necrotising pancreatitis: a 20-year experience of percutaneous drainage in a single centre. Dig Liver Dis. 2011; 43:478–483.

3. van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010; 362:1491–1502.
4. Working Group IAP/APA Acute Pancreatitis Guidelines. APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013; 13:4 Suppl 2. e1–e15.
5. Gerzof SG, Banks PA, Robbins AH, et al. Early diagnosis of pancreatic infection by computed tomography-guided aspiration. Gastroenterology. 1987; 93:1315–1320.

6. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013; 62:102–111.

7. Oana S, Shibata S, Matsumoto T. Pancreatico-colonic fistula after endoscopic ultrasound-guided cyst drainage for pancreatic pseudocyst: report of three cases. JOP. 2016; 17:334–339.
8. Will U, Meyer F, Hartmeier S, Schramm H, Bosseckert H. Endoscopic treatment of a pseudocystocolonic fistula by band ligation and endoloop application: case report. Gastrointest Endosc. 2004; 59:581–583.

9. Karvonen J, Gullichsen R, Salminen P, Grönroos JM. Endoscopic treatment of pseudocystocolonic fistula with fibrin glue. Gastrointest Endosc. 2010; 72:664–665.

10. Wille-Jørgensen P, Frederiksen HJ. Colonic necrosis or fistula following pancreatitis or gastric surgery. Eur J Surg. 1991; 157:137–139.
11. Suzuki A, Suzuki S, Sakaguchi T, et al. Colonic fistula associated with severe acute pancreatitis: report of two cases. Surg Today. 2008; 38:178–183.

12. Shatney CH, Sosin H. Spontaneous perforation of a pancreatic pseudocyst into the colon and duodenum. Am J Surg. 1973; 126:433–438.

13. Clements JL Jr, Bradley EL 3rd, Eaton SB Jr. Spontaneous internal drainage of pancreatic pseudocysts. AJR Am J Roentgenol. 1976; 126:985–991.

14. Jiang W, Tong Z, Yang D, et al. Gastrointestinal fistulas in acute pancreatitis with infected pancreatic or peripancreatic necrosis: a 4-year single-center experience. Medicine (Baltimore). 2016; 95:e3318.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download