Abbreviations
APC
CBA
CCR
CRD
FSC
GI
MMP
MyD88
PVDF
SSC
TIR
INTRODUCTION
MATERIALS AND METHODS
Purification of PBMCs and monocytes
Western blot analysis
Cytokine profile analysis using cytometric bead array (CBA) system
Measurements of cytokine production using ELISA
Confocal microscopy
Flow cytometry analysis
Co-immunoprecipitation
Phagocytosis assay
RESULTS
Gal-4 prevented the apoptosis of monocytes without affecting their proliferation
Figure 1
Effects of Gal-4 on apoptosis and proliferation of monocytes. (A) Human monocytes (5×105) were treated with 10 µg/ml of Gal-4. After 24 h, the intracellular levels of active caspase-3 were analyzed by flow cytometry. Cell apoptosis was evaluated by staining with FITC-conjugated annexin V and PI. Data are representative results of 3 independent experiments. (B) Human monocytes (1×105) were incubated with [3H] thymidine in the absence or presence of Gal-4 (10 µg/ml). At 24, 48, or 72 h post-treatment, [3H] thymidine incorporation was evaluated. Data are shown as mean ± standard deviation of 3 independent experiments. N.S, not significant.

Gal-4 altered the phenotypic characteristics of monocytes
Figure 2
Immunophenotypic characteristics of Gal-4-treated monocytes. (A-C) Human PBMCs were treated with Gal-4 (10 µg/ml) or LPS (1 µg/ml) for 24 h. (A) Expression levels of CCR1, CCR2, CXCR4, and CCR5 on CD14+ monocytes were analyzed by flow cytometry. (B) Expression levels of TLR1, TLR2, and TLR4 on the CD14+ monocytes were analyzed by flow cytometry. (C) Bars show expression levels of CD80, CD86, CD40, and HLA-DR on CD14+ monocytes. (A, C) Each value is mean±standard deviation of 3 independent experiments. (B) Data are representative results of 3 independent experiments.

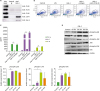
Gal-4 modulated the function of monocytes and induced their differentiation
Figure 3
Functional and morphological changes in Gal-4-treated monocytes. (A, B) Human monocytes (1×105) were treated with Gal-4 (10 μg/ ml) or LPS (1 μg/ml) for 24 h. Culture supernatant was harvested and assayed for TNF-α, IL-6, and IL-10 by CBA, and IL-12 and IL-1β by ELISA. (C) Human monocytes (5×105) were treated with Gal-4 (10 µg/ml) or LPS (1 µg/ml) for 48 h. Phagocytic ability of monocytes was determined as described in materials and methods. (D) Western blot analysis for active MMP-2 in Gal-4-treated (10 µg/ml) monocytes. (E) Western blot result for active MMP2 normalized to GAPDH. (F) Laser confocal microscopy of a section through the center of human monocytes incubated with Gal-4 for 48 h. Scale bar represents 10 μm. (G) Human PBMCs were treated with Gal-4 (10 μg/ml) or LPS (1 μg/ml) for 24 h. Flow cytometry analysis for the expression of the monocyte/macrophage markers was performed. (A, B, E, G) The values are mean±standard deviation from 3 independent experiments. (C, D, F) Data are representative results of 3 independent experiments. MFI, mean fluorescence intensity.
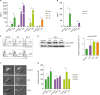
Gal-4 bound to CD14 and activated the MAPK signaling pathway in monocytes
Figure 4
Activation of MAPK signaling pathway after engagement of Gal-4 with CD14. (A) Interaction of Gal-4 and CD14 was analyzed by co-immunoprecipitation and western blot as described in materials and methods. (B, C) 2×105 human PBMCs were treated with Gal-4 (10 µg/ml) or LPS (1 µg/ml). To confirm the interaction of CD14 with Gal-4, cells were pretreated with anti-CD14 neutralizing Ab (10 µg/ml) or isotype control Ab (10 µg/ml) for 1 h before Gal-4 treatment. After 24 h, flow cytometry was performed and the monocyte cell subset was gated using FSC and SSC properties. (C) Cytokine production was estimated by CBA after 24-h Gal-4 treatment. The experiment was performed with 3 donor PBMCs, and triplicate data from a single donor is presented as representative data. Error bars indicate SD. (D) Human monocytes were treated with 10 µg/ml of Gal-4 for the indicated time periods. Representative western blots demonstrating phosphorylated ERK, p38, and JNK and total p38, JNK, and ERK. (E) The expression levels of phospho-p38, phospho-JNK, and phospho-ERK were normalized using total p38, JNK, and ERK. Data are representative results of 3 independent experiments.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download