Abstract
Background
The efficacy and safety of etanercept in the treatment of psoriasis has been proven, and the drug was approved for the treatment of moderate to severe psoriasis. However, there have been few studies that have presented real-world data focused on concomitant treatment during etanercept treatment, and the switching pattern after discontinuation of etanercept.
Objective
To reveal the real-world treatment pattern of etanercept-based psoriasis treatment and to investigate the switching pattern after withdrawal of etanercept.
Methods
We enrolled 66 patients with psoriasis who were treated with etanercept. We collected data regarding the demographic characteristics of the patients, etanercept treatment schedules, and other treatments administered during the etanercept treatment period. We also investigated the treatment pattern after the discontinuation of etanercept with emphasis on the drug-free interval and the administered treatment modalities.
Results
The mean treatment duration was 22.7±26.1 months and the mean number of etanercept injections was 21.5±27.9. Thirty-six patients were administered concomitant systemic medication or phototherapy. After discontinuation of etanercept, 54 patients were followed up and 34 of these patients were administered other systemic medication or phototherapy; phototherapy and cyclosporine was the most commonly administered treatment modality and 27.4% of treatments used biologics.
Conclusion
The treatment schedule for etanercept was modified according to the severity of psoriasis and concomitant treatment was administered to improve the effectiveness of treatment in the patients enrolled in the study. We also found that most patients required other treatment modalities to control psoriasis during the period of etanercept treatment.
Psoriasis is a chronic inflammatory skin disorder that is associated with a significant decline in the quality of life of patients1. The chronic and recurrent nature of psoriasis often requires long-term continuous treatment23. As the understanding of the role of the immune mechanism in psoriasis has increased, new biologics have been introduced in the treatment14. In Korea, infliximab, etanercept, adalimumab, ustekinumab, and secukinumab are available for the treatment of moderate to severe psoriasis. Etanercept is a fully human tumor necrosis factor (TNF)-α receptor protein that competitively inhibits the binding of TNF-α to the cell surface receptors, thereby preventing the activation of the TNF-α-mediated inflammatory cascade in patients with psoriasis14. The efficacy and safety of etanercept has been proven in the treatment of psoriasis, and it has been approved for the treatment of moderate to severe psoriasis since 2004 in Korea14. The utility of etanercept as an effective form of treatment for moderate to severe psoriasis has been investigated through various clinical trials; however, there have been few studies presenting real-world data focused on the switching pattern of treatment modalities after the discontinuation of etanercept. Previously, we reported the effectiveness of a low-dose etanercept therapy combined with systemic medication or phototherapy in Asian patients with non-severe psoriasis in comparison to that in a Western population1. In addition, other studies have reported the effectiveness of an intermittent etanercept treatment or a combination treatment with etanercept and acitretin for patients with plaque-type psoriasis24. Based on these previous studies, it can be inferred that etanercept is used with modified treatment regimens and in combination with other treatment modalities. However, there have been very few studies focused on concomitant systemic medication or phototherapy. Therefore, we investigated the effectiveness of concomitant treatment during etanercept treatment.
Newly introduced biologics have been reported to have better efficacy and the treatment schedule is simpler than what is used for etanercept-based treatment. Moreover, currently, etanercept is not frequently used for the treatment of psoriasis. A study on the pattern of etanercept treatment may reflect the need for treatment of patients with psoriasis. Such a study would allow us to anticipate the therapeutic pattern of newly developed biologics. Therefore, we performed an observational study to investigate the real-world experience of etanercept-based psoriasis treatment. This study is expected to reveal the essential elements of a good treatment modality for psoriasis.
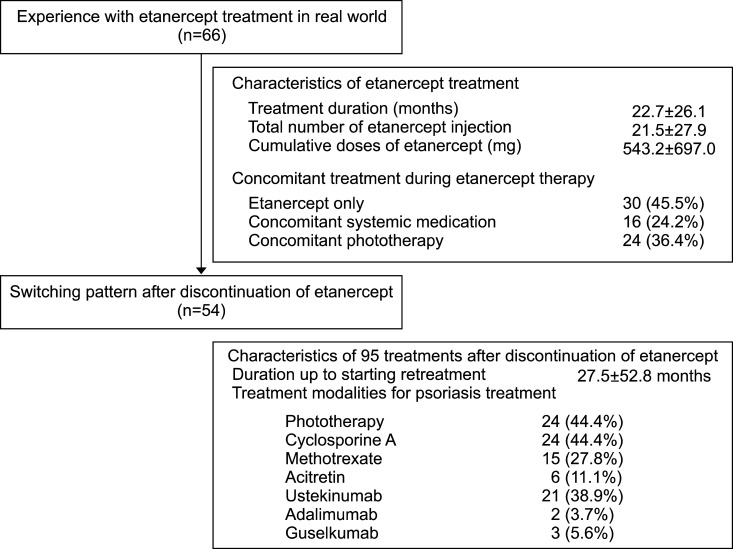
We enrolled 66 patients with psoriasis who were treated with etanercept between September 2004 and January 2016 at Seoul National University Bundang Hospital. Data on the demographic characteristics of the patients (comprising of information on age, sex, body mass index, comorbidity, duration of psoriasis, previous treatments for psoriasis, and biologics naivety), data related to the etanercept treatment (including information on other treatment modalities introduced during the treatment), and information regarding the treatment pattern after etanercept discontinuation (with emphasis on the drug-free interval and the administrated treatment modalities) were collected and analyzed to determine the characteristics and the real-world experience of etanercept treatment and the switching pattern after etanercept discontinuation. We enrolled 66 patients with psoriasis who were treated with etanercept between September 2004 and January 2016 at Seoul National University Bundang Hospital (Fig. 1).
The treatment regimen of etanercept in most patients enrolled in this study was modified from the standard regimen. The treatment regimen for the enrolled patients was initiated as a twice-weekly injection of etanercept. The dose and interval of etanercept injection was changed according to the patient's response to the treatment. The majority of patients were treated intermittently and the intervals between the injections were not consistent. Because some of the enrolled patients used etanercept intermittently, we defined the duration of the etanercept treatment period as between the date of first injection of etanercept and the date of last injection. Furthermore, concomitant treatment was defined as the use of other psoriasis treatment modalities, which were administered during the etanercept treatment period.
This study was approved by the institutional review board of Seoul National University Bundang Hospital (IRB number: B-1603/340-105).
Statistical analyses were performed using the Student t-test for comparing continuous variables. The chi-square test and Fisher's exact test were used for the analysis of categorical variables. Results were expressed as the mean±standard deviation (SD). We considered a p-value of <0.05 as statistically significant. Statistical analysis was performed using the IBM SPSS Statistics ver. 24.0 (IBM Co., Armonk, NY, USA).
The mean±SD age and duration of psoriasis at the initiation of etanercept treatment for the 66 enrolled patients with psoriasis was 52.8±15.4 years and 13.3±12.2 years, respectively (Table 1). Among these patients, chronic liver disease was found in 7 (10.6%) patients and was the most common comorbidity, followed by hyperlipidemia (5 [7.6%] patients) and hypertension (5 [7.6%] patients). Before undergoing etanercept treatment, 40 (60.6%) patients had undergone phototherapy, and 13 (19.7%) and 12 (18.2%) patients had received acitretin and cyclosporine for the treatment of psoriasis, respectively. Sixty-four (97.0%) patients were biologics-naïve, and the remaining two (3%) had used biologics: one patients had been administered ustekinumab and adalimumab, while the other had been administered efalizumab. Among the 66 patients with psoriasis, etanercept was first injected in November 2004 and the last injection was performed in July 2015.
The mean duration of the etanercept treatment period was 22.7±26.1 months and the mean number of etanercept injections and the mean cumulative dose was 21.5±27.9 and 543.2±697.0 mg (Table 1). Among the 66 patients, 36 patients were administered concomitant systemic medication or phototherapy during the etanercept treatment period. At the time of etanercept initiation, two and 11 patients were already on cyclosporine treatment and phototherapy, respectively. For the rest of these patients, however, concomitant systemic medication or phototherapy was initiated after the initiation of etanercept treatment. Among them, 14 patients were treated with cyclosporine, 6 with methotrexate, 5 with acitretin, and 24 with phototherapy during the etanercept treatment period. After an average of 4.6±7.5 months of etanercept treatment, concomitant systemic medication or phototherapy was initiated for 23 patients for psoriasis treatment. At the time of etanercept discontinuation, a total of 13 patients were on concomitant systemic medication or phototherapy owing to the loss of etanercept effectiveness. Among them, 4 patients were treated with cyclosporine, 3 with methotrexate, and 1 with acitretin. In addition, 7 patients were on phototherapy when etanercept treatment was discontinued. Two patients were administered a combination therapy of cyclosporine and phototherapy. There were no adverse events in those patients despite the overlap of immunosuppressive therapy (etanercept with cyclosporine, or etanercept with phototherapy).
After discontinuation of etanercept, we followed up with 54 patients during the study period. The other 12 patients were lost to follow-up. Of the 54 patients, 34 patients were continuously treated at out hospital and remaining 20 were treated at other hospitals after discontinuation of etanercept for a while and came back to our hospital during the study period. To analyze the switching pattern after discontinuation of etanercept, we summarized the initial treatment of 34 patients who continuously treated at our hospital (Table 2). The mean time to initiation of new treatment modalities among 34 patients was 15.5±35.6 months. Cyclosporine treatment was the most commonly administered treatment modality (14; 41.2%), followed by ustekinumab (9; 26.5%) and phototherapy (5; 14.7%). During the follow-up period, we administered a total of 95 treatments in 54 patients (Table 3). Among the administered treatments, phototherapy (24; 44.4%) and cyclosporine (24; 44.4%) were the most commonly administered treatment modalities, followed by ustekinumab (21; 38.9%).
The treatment for 26 (27.4%) patients after etanercept discontinuation involved the use of biologics. To determine the factors that influence the patient's decision of using biologics for the treatment of psoriasis, we compared the epidemiologic characteristics of the patients and the characteristics of the etanercept treatment between those who chose biologics after etanercept discontinuation and those who did not (Table 4). Between the two groups, the proportion of patients who underwent phototherapy before etanercept was higher among those who chose biologics after the discontinuation of etanercept (p=0.046).
Etanercept is a fully human TNF-α receptor protein that competitively inhibits the TNF-α-mediated inflammatory cascade in patients with psoriasis14. The common regimen of etanercept for chronic plaque-type psoriasis in Korea involves a biweekly injection of 25 mg of etanercept12. In case of poor response, the dose of etanercept can be increased up to 50 mg2. However, modified etanercept treatment regimens, such as low-dose etanercept therapy or combination treatment involving etanercept and other systemic medications have been proposed to improve the effectiveness or lower the cost of etanercept treatment12. Previously, we suggested that a low-dose etanercept therapy combined with conventional treatment modalities could be an alternative and less-expensive treatment for the patients with psoriasis who do not responding well to conventional etanercept treatment1. In this study, we found that systemic medication or phototherapy were administered during the etanercept treatment period when the effectiveness of the etanercept treatment was not sufficient. In addition, we also found that systemic medication or phototherapy were used between the intermittent etanercept treatments. Among the 36 patients (54.5%) who were administered systemic medication or phototherapy during the etanercept treatment period, etanercept was initiated during the systemic medication or phototherapy in 13 patients. In contrast, concomitant systemic medication or phototherapy was initiated for the rest after initiation of etanercept treatment. The time interval between the initiation of etanercept treatment and the initiation of concomitant systemic medication or phototherapy was 4.6±7.5 months in 23 patients. The time interval suggested that single therapy with etanercept is unable to control the activity of psoriasis. In this study, we again confirmed that the etanercept therapy combined with conventional treatment modalities could be used effectively in Korean patients with psoriasis.
Since psoriasis involves the chronic relapsing inflammation of the skin, the combination treatment and rotational approach was introduced to achieve sustained disease control without the development of adverse events35. In this study, we found that a total of 36 patients were treated with concomitant systemic medication or phototherapy as combinational or rotational treatment during the etanercept treatment period. Among them, phototherapy was the most frequently administered treatment modality during the etanercept treatment period, followed by cyclosporine. This result is slightly different from what is reported in previous studies. Busard et al.6 investigated the conventional systemic agents or phototherapy combined with biologics and found that methotrexate was most frequently used compound. However, this study only analyzed the concomitant combined treatment during the use of biologics, whereas we investigated the combined treatment with etanercept and the rotational treatment between intermittent etanercept treatments. Considering the profuse experience in the combined use of methotrexate with biologics67, the results of previous studies and the results from ours will justifiably have some differences.
In a previous study performed in Germany, the mean drug-free intervals after etanercept discontinuation was 12.9 weeks4. This study also stated that 37.3% of patients who discontinued etanercept treatment switched to another anti-TNF agent4. In this study, we investigated the treatment pattern after etanercept discontinuation and analyzed the switching pattern. Among the enrolled patients, 34 patients initiated another treatment for psoriasis after 15.5±35.6 months and 10 of these patients (29.4%) chose other biologics. Compared to the previous study, this longer drug-free interval can be a result of the lower severity of psoriasis in Asian patients compared to Caucasians. Lastly, we have followed up 54 patients with psoriasis and found that 26 (48.1%) used biologics during the follow-up period. Among the other biologics used during the follow-up period, ustekinumab was the most commonly administered. To determine the factors that influence patients with psoriasis to choose biologics, we performed additional analysis and found that the proportion of patients who experienced phototherapy before starting etanercept was higher in the patients choosing other biologics after etanercept discontinuation. It may be inferred that the concern regarding the possibility of long-term adverse events resulting from the continued use of systemic medications might influence the choice of using phototherapy or biologics.
In choosing the treatment modalities, the severity of psoriasis can be a determining factor. As our study was a retrospective study using data from medical chart in real-world, assessing the Psoriasis Area and Severity Index (PASI) score was not performed in every visit. For this reason, we could not retrieve enough PASI scores for analysis and we are not able to analyze the effect of severity of psoriasis on the treatment pattern or switching pattern of psoriasis treatment.
In conclusion, for Asian patients with a milder form of psoriasis in comparison to Caucasians patients, etanercept therapy with concomitant systemic medication or phototherapy can be an effective treatment modality. Considering the introduction of new biologics with superior therapeutic efficacy into the treatment regimen of psoriasis89, the use of biologics in psoriasis treatment is expected to increase further and the combinational and rotational treatment with new biologics and conventional systemic medication or phototherapy will be introduced to improve the effectiveness of psoriasis treatment and reduce the economic burden associated with psoriasis treatment.
References
1. Na JI, Kim JH, Park KC, Youn SW. Low-dose etanercept therapy in moderate to severe psoriasis in Korean. J Dermatol. 2008; 35:484–490. PMID: 18789067.

2. Lee JH, Youn JI, Kim TY, Choi JH, Park CJ, Choe YB, et al. A multicenter, randomized, open-label pilot trial assessing the efficacy and safety of etanercept 50 mg twice weekly followed by etanercept 25 mg twice weekly, the combination of etanercept 25 mg twice weekly and acitretin, and acitretin alone in patients with moderate to severe psoriasis. BMC Dermatol. 2016; 16:11. PMID: 27455955.

3. Choi CW, Kim BR, Ohn J, Youn SW. The advantage of cyclosporine A and methotrexate rotational therapy in long-term systemic treatment for chronic plaque psoriasis in a real world practice. Ann Dermatol. 2017; 29:55–60. PMID: 28223747.

4. Luger T, Schopf RE, Schwanke A, Langhammer S, Meng T, Löschmann PA. An observational study to evaluate the long-term outcomes of treatment with etanercept in patients with plaque-type psoriasis. J Eur Acad Dermatol Venereol. 2016; 30:1730–1741. PMID: 27297981.

5. Lebwohl M, Menter A, Koo J, Feldman SR. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004; 50:416–430. PMID: 14988684.

6. Busard CI, Cohen AD, Wolf P, Gkalpakiotis S, Cazzaniga S, Stern RS, et al. Biologics combined with conventional systemic agents or phototherapy for the treatment of psoriasis: real-life data from PSONET registries. J Eur Acad Dermatol Venereol. 2018; 32:245–253. PMID: 28898541.

7. Zweegers J, Otero ME, van den, van Lümig PP, Driessen RJ, Kievit W, et al. Effectiveness of biologic and conventional systemic therapies in adults with chronic plaque psoriasis in daily practice: a systematic review. Acta Derm Venereol. 2016; 96:453–458. PMID: 26537336.

8. Kimball AB, Papp KA, Wasfi Y, Chan D, Bissonnette R, Sofen H, et al. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis treated for up to 5 years in the PHOENIX 1 study. J Eur Acad Dermatol Venereol. 2013; 27:1535–1545. PMID: 23279003.
9. Vergou T, Moustou AE, Antoniou C. Five-year experience with Ustekinumab for psoriasis: real-life data of a single centre. J Eur Acad Dermatol Venereol. 2017; 31:e40–e41. PMID: 27038363.

Table 1
Baseline demographics and characteristics of patients (n=66)
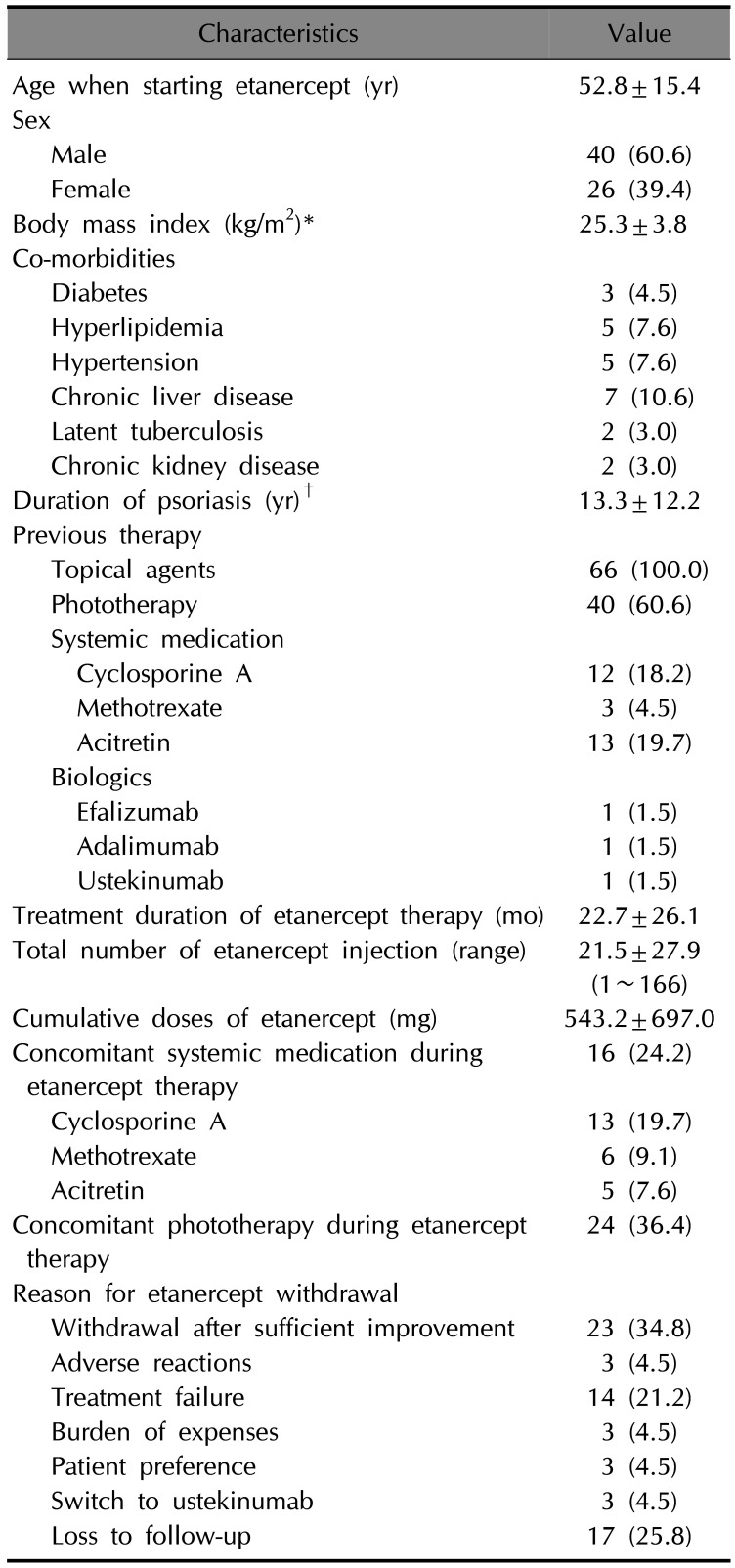
Table 2
Characteristics of initial treatment after the withdrawal of etanercept among the patients who continuously treated at our hospital (n=34)*
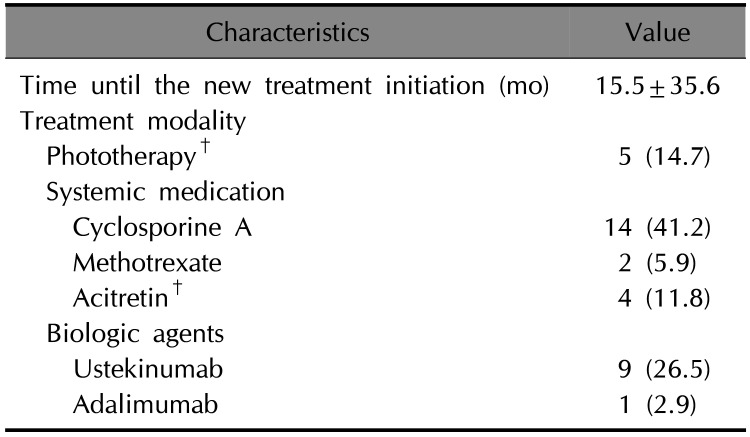
Table 3
Characteristics of 95 treatments after the discontinuation of etanercept (n=54)*
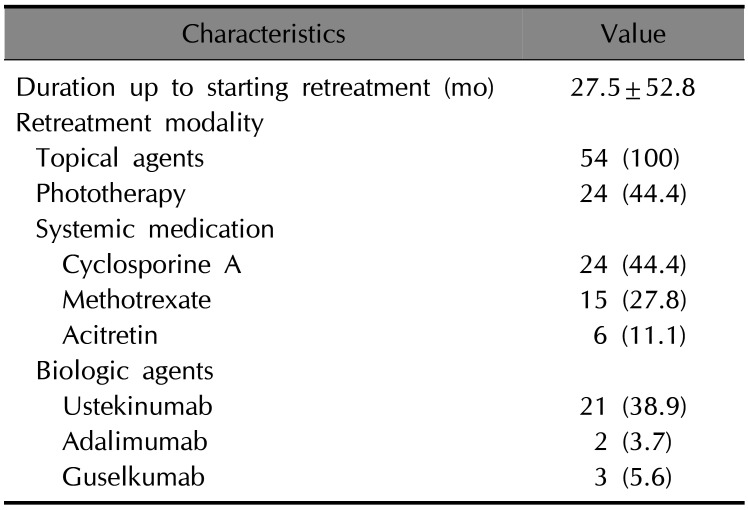
Table 4
Comparison between the patients who chose biologics after etanercept discontinuation and those who did not
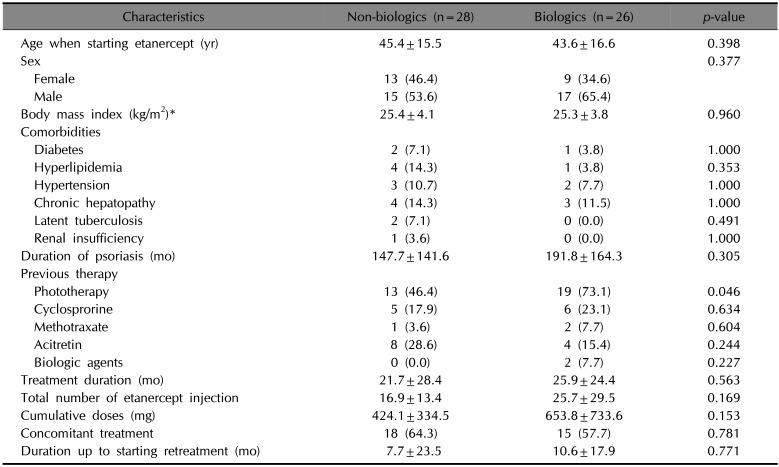




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download