1. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011; 123:e18–e209. PMID:
21160056.
2. Jackson CF, Wenger NK. Cardiovascular disease in the elderly. Rev Esp Cardiol. 2011; 64:697–712. PMID:
21723657.

3. American Thoracic Society. American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003; 167:211–277. PMID:
12524257.
4. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016; 4:607–616. PMID:
27289406.

5. Moran J, Wilson F, Guinan E, McCormick P, Hussey J, Moriarty J. The preoperative use of field tests of exercise tolerance to predict postoperative outcome in intra-abdominal surgery: a systematic review. J Clin Anesth. 2016; 35:446–455. PMID:
27871573.

6. Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J Am Coll Cardiol. 2002; 40:1531–1540. PMID:
12392846.
7. Keteyian SJ, Isaac D, Thadani U, Roy BA, Bensimhon DR, McKelvie R, et al. Safety of symptom-limited cardiopulmonary exercise testing in patients with chronic heart failure due to severe left ventricular systolic dysfunction. Am Heart J. 2009; 158(4 Suppl):S72–S77. PMID:
19782792.

8. Skalski J, Allison TG, Miller TD. The safety of cardiopulmonary exercise testing in a population with high-risk cardiovascular diseases. Circulation. 2012; 126:2465–2472. PMID:
23091065.

9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18:891–975. PMID:
27207191.
10. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129:2440–2492. PMID:
24589852.

11. Shapiro LM, McKenna WJ. Distribution of left ventricular hypertrophy in hypertrophic cardiomyopathy: a two-dimensional echocardiographic study. J Am Coll Cardiol. 1983; 2:437–444. PMID:
6683731.

12. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011; 124:2761–2796. PMID:
22068435.

13. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013; 128:873–934. PMID:
23877260.
14. Scardovi AB, Coletta C, De Maria R, Perna S, Aspromonte N, Feola M, et al. The cardiopulmonary exercise test is safe and reliable in elderly patients with chronic heart failure. J Cardiovasc Med (Hagerstown). 2007; 8:608–612. PMID:
17667032.

15. Myers J, Buchanan N, Walsh D, Kraemer M, McAuley P, Hamilton-Wessler M, et al. Comparison of the ramp versus standard exercise protocols. J Am Coll Cardiol. 1991; 17:1334–1342. PMID:
2016451.

16. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016; 374:1609–1620. PMID:
27040324.
17. Coats CJ, Rantell K, Bartnik A, Patel A, Mist B, McKenna WJ, et al. Cardiopulmonary exercise testing and prognosis in hypertrophic cardiomyopathy. Circ Heart Fail. 2015; 8:1022–1031. PMID:
26374874.

18. Sorensen LL, Liang HY, Pinheiro A, Hilser A, Dimaano V, Olsen NT, et al. Safety profile and utility of treadmill exercise in patients with high-gradient hypertrophic cardiomyopathy. Am Heart J. 2017; 184:47–54. PMID:
27892886.

19. Balady GJ, Jette D, Scheer J, Downing J. Changes in exercise capacity following cardiac rehabilitation in patients stratified according to age and gender. Results of the Massachusetts Association of Cardiovascular and Pulmonary Rehabilitation Multicenter Database. J Cardiopulm Rehabil. 1996; 16:38–46. PMID:
8907441.
20. Morris CK, Myers J, Froelicher VF, Kawaguchi T, Ueshima K, Hideg A. Nomogram based on metabolic equivalents and age for assessing aerobic exercise capacity in men. J Am Coll Cardiol. 1993; 22:175–182. PMID:
8509539.

21. Kim C, Choi HE, Lee KH, Kim YJ, Lee SJ. Influence of low peak respiratory exchange ratio on cardiac rehabilitation in patients with coronary artery disease. Ann Rehabil Med. 2016; 40:1114–1123. PMID:
28119843.

22. Boo SH, Joo MC, Lee JM, Kim SC, Yu YM, Kim MS. Association between skeletal muscle mass and cardiorespiratory fitness in community-dwelling elderly men. Aging Clin Exp Res. 2019; 31:49–57. PMID:
29916089.

23. Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002; 31:119–125. PMID:
11937474.

24. Quinn TJ, Smith SW, Vroman NB, Kertzer R, Olney WB. Physiologic responses of cardiac patients to supine, recumbent, and upright cycle ergometry. Arch Phys Med Rehabil. 1995; 76:257–261. PMID:
7717819.

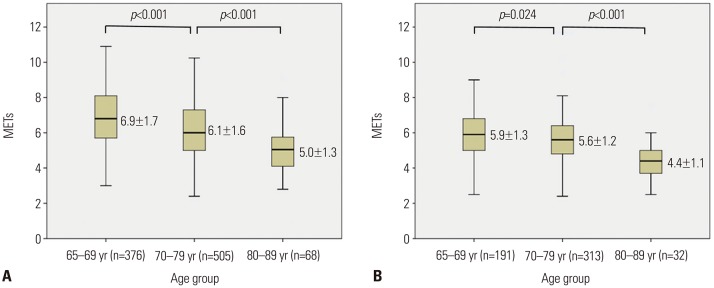
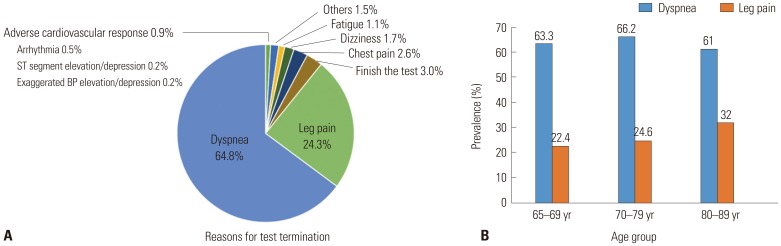
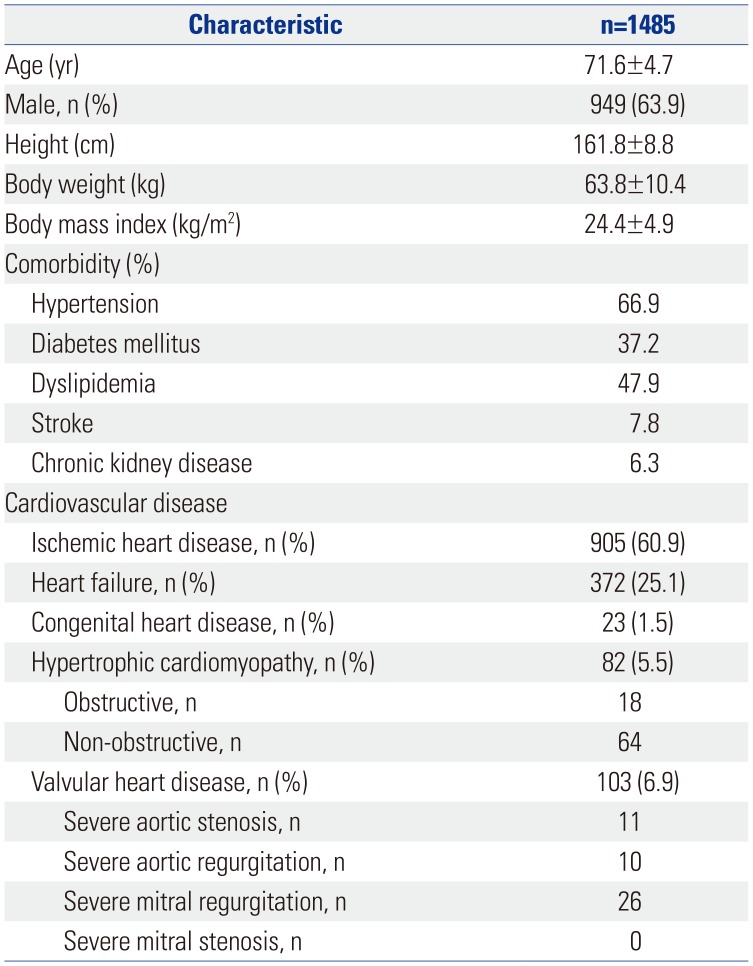
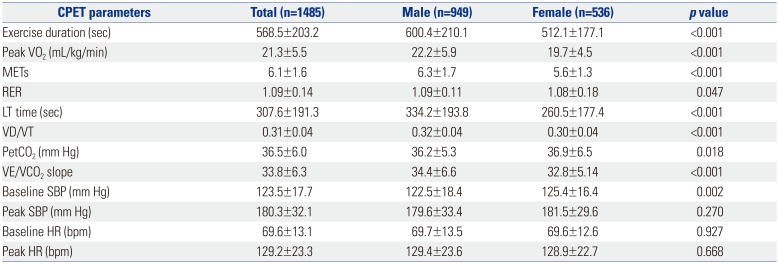




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download