INTRODUCTION
Spinal hemorrhage itself is rare and most of it is epidural hematoma associated with trauma.
3) Most reported cases of spinal subdural hematoma (SDH) and spinal subarachnoid hemorrhage (SAH) are iatrogenic causes such as trauma or spinal puncture.
13) The patients with spontaneous spinal SDH or SAH tend to have a bleeding tendency like thrombocytopenia, anticoagulation medication or antiplatelet agent medication.
12356) There have been several reports of spinal SDH or SAH associated with intracranial hemorrhage.
256) The types of accompanied intracranial hemorrhage were SAH or chronic SDH.
256) There are few reports of acute form spinal SDH in the literature, and there is a great difference in the clinical course and prognosis of the subacute form.
156) We report a case of spinal SDH & SAH with concomitant intracerebral hemorrhage (ICH) that has not been reported yet with a review of the literature.
CASE REPORT
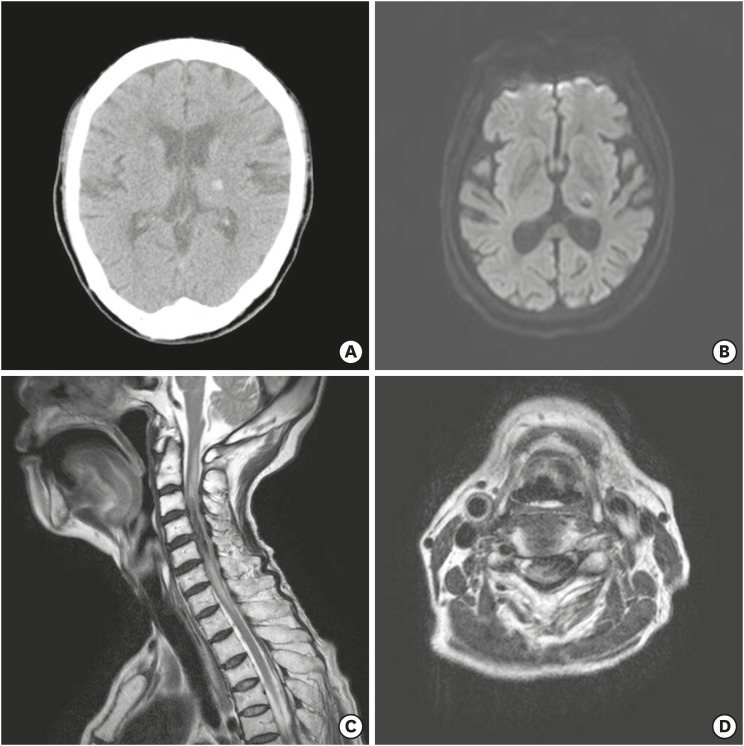
A 74-year-old female patient came to the emergency room with left side weakness. The patient had been taking aspirin and warfarin as she has had a history of hypertension for 30 years, had cerebral infarction 10 and 5 years ago. There was no traumatic history prior to the onset of pain. Initial neurological examination showed left side hemiparesis with a grade III on upper extremity and a grade IV on lower extremity. International normalized ratio was prolonged to 2.13 with laboratory results and there was no other bleeding tendency. Initial brain computed tomography (CT) showed small ICH in the left side thalamus but the patient's clinical symptoms did not match (
FIGURE 1A). Additional brain magnetic resonance image (MRI) & magnetic resonance angiography (MRA) did not reveal acute lesion that could cause left side hemiparesis besides ICH of the left thalamus on Brain CT (
FIGURE 1B). After 4 hours, the patient's hemiparesis more worsened to grade I on upper limb and grade II on lower limb. Cervical spine MRI showed the intradural hematoma located in the left side ventral portion of C3-4-5 level (
FIGURE 1C & D).
FIGURE 1
(A) Noncontrast brain computed tomography showed acute intracerebral hemorrhage in the left side thalamus. (B) Brain magnetic resonance diffuse weighted image showed no acute cerebral infarction. Magnetic resonance image of (C) sagittal T2 weighted and (D) axial T2 weighted on the C5 level showed intradural acute hemorrhage extending from C3 to C6.
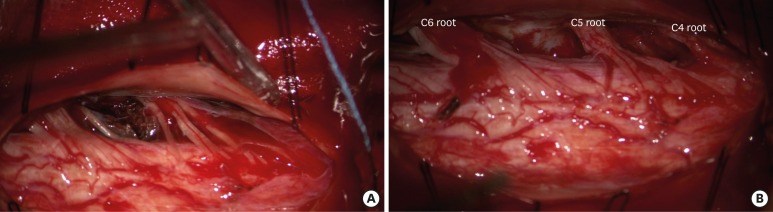
We performed C3-4-5 total laminectomy and opened dura longitudinally. Small spinal SDH was observed and the spinal SAH had much clotted hematoma interposed among the left C3-4-5 rootlets (
FIGURE 2A). We removed as much of the hematoma as possible to avoid damaging the rootlets (
FIGURE 2B). After the surgery, the muscle strength was partially improved on upper extremity with grade II and lower extremity with grade II. 2 weeks later, the patient was transferred to the rehabilitation department for rehabilitation. There was no improvement in the left hemiparesis of the patient after one year.
FIGURE 2
(A) Intraoperative photographic images under a microscope: after total laminectomy on C3-6, the dura and arachnoid membrane was longitudinally opened, which showed small CSF-mixed hematoma in subdural space and a dark blood color of hematoma in the subarachnoid space between the dorsal & ventral nerve roots of the right side 3rd to 6th cervical root and compressed spinal cord. (B) After total removal of hematoma, there was no specific bleeding focus, and the Dura was repaired after sufficient decompression of the hematoma.
CSF: cerebrospinal fluid.
DISCUSSION
Spinal SDH is very rare, accounting for approximately 4% of total spinal hemorrhages, and the cause is strongly associated with trauma.
3) The conditions that can cause spinal hemorrhage include trauma, surgery, vascular malformation, underlying neoplasm, spinal puncture, acquired or congenital coagulopathy.
123456) In particular, acquired coagulopathy is associated strongly with an increasing use of anticoagulants and antiplatelet agents.
12356) Most of the spontaneous spinal SDH reported so far are accompanied by coagulopathy.
12356) In the cases of acute form, the mechanism of bleeding is presumed to be due to sudden increase in intraabdominal or intrathoracic pressure in patients with a risk factor for bleeding, resulting in elevation of pressures in the internal vertebral venous plexus and venous bleeding.
13)
Anatomically, because the spinal subdural space is very narrow and there is avascular area that lacks the bridging veins compared with the cranial subdural space, the spinal SDH cannot occur alone.
13) Because the epidural hematoma is difficult to penetrate the dura, in the case of spontaneous spinal SDH, the cause of hemorrhage occurs in the subarachnoid space and is thought to be extended through the arachnoid membrane into the subdural space.
13) On the MRI scan, the SDH and SAH of the lumbar spine can be distinguished, but in the cervicothoracic region, the two spaces are too narrow to distinguish.
3) It is very difficult to distinguish between spinal SDH and SAH by radiologic examination alone, which can be confirmed during surgical treatment.
5) In our case, hematoma was observed in both subdural and subarachnoid space and SAH was much observed. If anatomically only acute spinal subdural hematoma occurs, it can be assumed that some of the vessel in the subarachnoid space are located in the elongated arachnoid membrane protruding toward the subdural space, which result in bleeding into the subdural space.
1)
There are a few reports accompanied by spontaneous spinal SDH and intracranial hemorrhage.
2456) It is not known how the occurrence of concomitant intracranial and spinal hemorrhage is related. However, in some cases, the cranial SDH has been extended to the spinal subdural space, which is considered to be extended in one space.
6) In case of spinal SDH associated with cranial subacute SDH, MRI showed that the stage of cranial and spine hemorrhage was similar to each other.
4) This may be presumably because cranial and spinal hemorrhages occurred at similar times. Most cases of spinal SDH with symptoms also improved with burrhole trephination alone for cranial subacute SDH.
4) But, clinical features of acute form spinal SDH and chronic form SDH are different.
456) Spinal lesions in the chronic form of SDH are mostly localized to the lumbar spine then because most of symptoms of spine lesion are improved by conservative treatment, most of them is not need to surgical treatment.
4)
However, in the case of acute hemorrhage, surgical treatment is required for the spinal part as in our case, it is necessary to have early diagnosis and treatment because it can cause sequelae.
56) In the case of acute spinal SDH or SAH, if bleeding rate is slow, the blood clots are not formed due to mixing it with the cerebrospinal fluid (CSF), and may not compress the nerves as the rostro-caudal expansion occurs.
3) But, if the CSF flow is slow, or if the amount of bleeding rapidly increases in a closed situation, blood is clotted without mixing with CSF, causing a mass effect or penetrating dura and forming subdural hematoma.
3) In addition, if this phenomenon is localized to either side, hematoma is also biased toward one side, which may appear as a symptom of cranial lesion such as hemiparesis, which may lead to delayed diagnosis of the lesion and delayed treatment resulting in neurological deficit.
356) In our case, the symptom of hemiparesis is not related to ICH, but with the judgment of the false localization sign (Kernohan-Woltman notch phenomenon), brain MRI was performed first and the diagnosis of spinal hemorrhage was delayed, and it is closely related to the prognosis of the patient.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download