Abstract
Purpose
Methods
Results
Figures and Tables
Fig. 1
Patients selection. PD, pancreaticoduodenectomy. a)Percent-age of patients who received intervention. b)Success rate among 62 patients who underwent intervention.
Fig. 3
(A) Short-term primary endpoint. (B) Long-term primary endpoint. EMB, embolization only; STENT, stent-graft placement only. a)Number of cases that both procedures were done simultaneously or different times. b)Number of patients except those who died due to short-term complications.

Fig. 4
Patient data. A series of images in cases of long-term stent occlusion. This example is from a 62-year-old woman with pancreatic cancer who underwent pylorus-preserving pancreaticoduodenectomy. (A) Celiac angiography demonstrated a pseudoaneurysm at broad segment of artery along the proper hepatic artery and common hepatic artery (red arrows) on postoperative day 20. (B) Completion angiogram after placement of the stent-graft (white arrows) showed complete exclusion of ruptured site without remnant bleeding. The left hepatic artery received blood flow (black head of arrows) from intraheapatic collateral (red head of arrows) and left gastric artery (LGA, yellow head of arrows). (C) Follow-up CT scan taken 4 days after intervention demonstrated normal liver parenchymal perfusion due to collateral from the LGA (red arrows). (D) Twenty-one months after intervention, the stent occlusion (red arrows) was found on follow-up CT scan. The most part of liver parenchyma was normal.
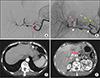
Table 1
Clinicopathologic characteristics of enrolled patients (n = 57)

Values are presented as median (range) or number (%).
PPPD, pylorus preserving pancreaticduodenctomy; PRPD, pylorus resecting pancreaticoduodenectomy; POPF, postoperative pancreatic fistula; GDA, gastroduodenal artery; PHA, proper hepatic artery; CHA, common hepatic artery; SMA, superior mesentery artery; LHA, left hepatic artery, RHA, right hepatic artery; RHA br., branch of RHA.
a)One ampulla of vater adenoma case, 1 ampulla of vater neuroendocrine carcinoma case, 1 common bile duct adenoma case, 1 low grade intraductal papillary mucinous neoplasm case, 1 intraductal papillary neoplasm of bile duct case. b)Number of event that pseudoaneurysmal rupture developed and it overlaps in patients. c)One case of pancreatic branch of splenic artery; 1 case of pancreaticoduodenal arcade, respectively.
Table 2
Patterns of collateral pathways formation

‘N’ indicate number of patients according to different types of intervention. ‘N’ implies number of cases as follows; (1) Collateral vessels have already existed at the time of intervention or (2) Development of collateral vessels are detected on follow up CT after successful intervention.
GDA, gastroduodenal artery; PHA, proper hepatic artery; CHA, common hepatic artery; LHA, left hepatic artery; RHA, right hepatic artery; LGA, left gastric artery; RGA, right gastric artery; IPha, infraphrenic artery; IHc, intrahepatic collateral; rRHA, replaced right hepatic artery; rLHA, replaced left hepatic artery; Acc LHA, accessory left hepatic artery; RHA-PB, right hepatic artery posterior branch; SMA br., branch of SMA; SA br., pancreatic branch of splenic artery.
a)Occurrence of rebleeding at a different site than before. b)At first try, stent-graft insertion was done at CHA excluding rupture site of GDA. In second try, pseudoaneurysm developed at PHA adjacent from end of stent having been inserted previously.
Table 3
Data about patients with long-term stent-graft related complications

IAI, intraabdominal infection; GDA, gastroduodenal artery; PHA, proper hepatic artery; CHA, common hepatic artery; LHA, left hepatic artery; RHA, right hepatic artery; LGA, left gastric artery; RGA, right gastric artery; IPha, infraphrenic artery; IHc, intrahepatic collateral; rRHA, replaced right hepatic artery; rLHA, replaced left hepatic artery; AccLHA, accessory left hepatic artery; RHA-PB, right hepatic artery posterior branch; SMA br., branch of SMA; SA br., pancreatic branch of splenic artery; NA, not available.
a)Presence of bifurcation or branches near rupture site (within 5 mm). b)Two stents were used simultaneously. c)At first try, stent-graft insertion was done at CHA due to pseudoaneurysm formation at GDA stump portion. After 2 days, rebleeding developed around proximal margin of stent-graft and stent-graft was inserted at PHA covering re-bleeding site successfully.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download