Abstract
Purpose
We evaluated the impact of collagenase clostridium histolyticum (CCH) on rates of diagnosis, treatment, and corporal rupture in Peyronie's disease (PD). We examined the impact of CCH on cost of PD treatment.
Materials and Methods
We extracted data on PD diagnosis (ICD-9 607.95 and ICD-10 N48.6), corporal rupture (ICD-9 959.13 and ICD-10 S39.840A), CCH use (J0775), penile injections (CPT 54200), and corporal rupture repair from 2008 to 2016 in men over 40 years old using the Clinformatics® Data Mart Database (3.7 to 4.9 million males). We analyzed for prevalence of PD, rates of PD treatments, cost associated with treatment, and rates of corporal rupture and repair by year.
Results
The prevalence of PD was 0.29% in 2013 and did not increase after CCH entered the market in 2014. An average of 2.52% of men with PD received treatment before CCH, compared with 3.75% after (p<0.0001). Penile injection rates increased (1.34% vs. 2.61%, p<0.0001), while rates of surgical treatments decreased between these periods. There was no change in rate of corporal rupture in men with PD before (0.024%) and after (0.024%) CCH. Overall, only 20.0% of corporal ruptures were repaired. After CCH entered practice, a significant increase in cost occurred (p=0.013).
Conclusions
The prevalence of men with PD did not change after CCH. However, more men with PD received treatment due to an increase in penile injections. The cost of treating PD increased after CCH became available. The overall prevalence of corporal rupture did not change after CCH entered the market.
Peyronie's disease (PD) is characterized by fibrous penile plaque formation in the tunica albuginea, leading to penile deformities that may interfere with sexual function [1]. When left untreated, the disease has a variable course with some patients spontaneously improving, and others progressing to significant disability. The prevalence of PD has been estimated to be between 0.5% and 20.3% [23], though there is concern these may be underestimates given the potentially embarrassing nature of the condition. While diabetes and erectile dysfunction have been postulated as medical comorbidities [34], PD has been linked to depression, low self-esteem, and emotional distress [5].
A number of therapies for PD exist. Of these, only intralesional verapamil, intralesional collagenase clostridium histolyticum (CCH), tunical plication, plaque incision/excision, and penile prosthesis are currently recommended by American Urological Association (AUA) guidelines [1]. In particular, there has been significant interest in use of CCH given the weak evidence for efficacy of verapamil [167] and the invasive nature of surgical procedures. Indeed, phase III studies showing significant improvements in penile curvature and PD symptoms with CCH led to approval from the United Stated Food and Drug Administration in 2013 [8]. Given the impressive results of initial CCH trials, there has been a substantial increase in PD research. In 2015 and 2016, there were 103 and 102 “Peyronie's Disease” PubMed articles indexed, compared with 68, 86, 68, and 64 in the 4 years prior. However, if any changes in the rates of diagnosis and management of PD have occurred in tandem remains unknown.
Despite its potential as a therapy, CCH has been associated with significant adverse events. An analysis of the data from both phase III clinical trials of CCH found that 84.2% of patients in the CCH group had an adverse event, compared to 36.3% in the placebo arm. Though most adverse events were mild or moderate (79.0%), 3 corporal ruptures and 3 severe hematomas occurred in the CCH group [8]. Subsequent studies have reported that 34% of urologists administering CCH have encountered a corporal rupture [9], and in one series, 4.9% of patients treated with CCH developed this complication. In the later study, 20% of cases were managed non-operatively [10]. However, to date, there have been no studies looking at population-level data to assess if corporal rupture rates have increased in the era of CCH use or compared CCH corporal rupture rates to other injection agents used in practice.
The primary goal of this study was to use a national insurance claims database to define the prevalence of PD, utilization of AUA recommended treatments for PD, and cost of treating PD in actual practice and examine how CCH availability has affected these. Further, we assessed the rates of corporal rupture and repair in men with PD and association with CCH availability and use.
The study protocol was approved by the Institutional Review Board of Stanford University (IRB# 35751). Informed consent was not necessary given use of a deidentified and HIPAA (Health Insurance Portability and Accountability Act) compliant database.
Optum Clinformatics® Data Mart Database is a deidentified and HIPAA compliant database from a large national insurance provider that stores data from adjudicated and paid insurance claims. We identified all men of 40 years of age or older, insured from 2008 to 2016, and used this as our study population. A cutoff of 40 years-old was selected to focus on age groups where PD is most common. Between 3.7 and 4.9 million males were covered annually during the study period. These individuals represent a geographically and ethnically diverse population from multiple age groups. Data collected includes patient demographic characteristics, international classification of diseases (ICD-9 and 10) codes, and current procedural terminology (CPT) codes. This database has been used in studies across the medical spectrum including the fields of internal medicine [11], endocrinology [12], and gastroenterology [13], among others.
For men in our study population, we extracted codes related to PD diagnosis (ICD-9 607.95 and ICD-10 N48.6) and treatment (CPT 54200, 54205, 54300, 54304, 54360, 54110, 54111, 54400-54405). These CPT codes map only to AUA-recommended PD therapies. Use of oral agents and other therapies not recommended in the AUA guidelines were excluded from analysis. Code J0775 (a Healthcare Common Procedure Coding System code) was used to determine CCH use. Use of CCH to treat PD was confirmed by requiring both a CPT code for penile injection and J0775.
We counted the number of men who had a PD diagnosis in each year and used this to determine prevalence. Similarly, we counted the number of men receiving treatments in each year and used these to calculate treatment rates. Both individual years and eras before and after CCH use (2008–2013 vs. 2014–2016) were analyzed for prevalence of PD, overall rate of PD treatment, rate of penile injections, and rate of PD surgical procedures. We extracted paid, adjudicated claims associated with PD treatment, and used these to calculate cost associated with PD treatment. Costs associated with diagnosis of PD were not included in our analysis, but all costs for the complete follow-up interval of every treatment were included. Given the range of years covered in the study, all costs were inflation adjusted.
For the same men, we extracted codes related to corporal rupture (ICD-9 959.13 and ICD-10 S39.840A) and repair (CPT 54437 and 54440). Corporal ruptures were only identified as being related to penile injections if they occurred after at least 1 penile injection was administered (time from last injection to rupture reported in days). We then analyzed data for rates of corporal rupture and repair by era, number of ruptures after CCH injections, and number of ruptures after non-CCH penile injections.
Prevalence of diagnoses and rates of treatments were calculated by dividing the number of men with the characteristic of interest by the entire study population within each year or time period. In regards to PD diagnosis and treatment, we compared the prevalence of PD diagnosis, rates of penile injections, rates of PD surgical procedures, and median cost of PD treatment per man before and after CCH entered the market (2008–2013 vs. 2014–2016). For corporal rupture, we compared the prevalence of corporal rupture and rates of corporal rupture repair before and after CCH entered the market. Further, we compared the rate of corporal rupture after CCH administration in 2014 to 2016 to other injected agents used in 2008 to 2013 (e.g., verapamil and interferon). For this specific comparison we excluded men who received treatments in both eras. This was done in order to ensure men that received CCH from 2014 to 2016 had not also received penile injections with other agents. The chi-square test was used to compare prevalence and rates and Wilcoxon rank-sum test was applied to compare costs. All tests were two-sided and p<0.05 was considered statistically significant. All analyses were conducted using SAS Institute Inc. (ver. 9.4; Cary, NC, USA).
An overview of our cohort is seen in Table 1. The annual prevalence of men with PD rose from 0.09% in 2008 to 0.29% by 2013 for the entire cohort. From 2014 to 2016, prevalence remained stable, ranging between 0.29% and 0.30%. PD was most prevalent in men 50 to 59 years old (0.11% to 0.38%) and 60 to 69 years old (0.16% to 0.48%) during the study period (Fig. 1).
The annual percentage of men with PD who received treatment ranged between 2.20% and 2.36% for 2008 to 2013 (average 2.52% of men with PD treated). The rate of treatment increased to 3.36% in 2014 and further increased to 3.77% in 2015 and 4.04% in 2016 (average 3.75% of men with PD treated). The increase in average rate of PD treatment between eras was significant (p<0.0001). Corresponding to the increase in treatment, average rates of penile injection increased from 1.34% for 2008 to 2013 to 2.61% for 2014 to 2016 (p<0.0001). Average rates of penile plication (0.58% vs. 0.51%, p=0.11), penile grafting (0.22% vs. 0.13%, p=0.002), and penile prosthesis (0.68% vs. 0.58%, p=0.04) appeared to decrease when comparing the same periods (Table 2).
The median annual inflation-adjusted cost of PD treatment rose from $5,832 per man in 2008 to $10,022 per man in 2016. While costs were overall stable between 2008 to 2013 (range, $2,960–$5,832), they sharply increased after CCH became available in 2014. The median cost of PD treatment increased by $5,286 from 2013 (median, $2,960) to 2014 (median, $8,246). The median cost continued to increase after, reaching $10,022 in 2016. Comparing the years before and after CCH entered the market, the increase in cost was significant (p=0.01, Table 2).
A total of 25 corporal ruptures occurred in men with PD during the study period. When comparing before and after CCH entered the market, there was no significant change in prevalence between the eras (0.024% vs. 0.024%, p<0.999). Additionally, only 20% of corporal ruptures were repaired in both eras.
To our knowledge, this is the first study to examine how CCH has affected AUA-recommended management of PD in actual practice. We found that the reported prevalence of men with PD did not change after CCH entered the market. However, we did find that more men with PD received treatment due to a clinically and statistically significant increase in penile injection utilization. Though the cost of treating PD per man increased over the entire study period, this was most pronounced after CCH became available. Given the significant increase in penile injections during this period and substantial cost associated with CCH use [14], the sharp rise is likely due to CCH use. We found that risk of corporal rupture in men with PD is low, and the overall prevalence of corporal rupture did not change after CCH entered the market.
In our population, the prevalence of PD remained at approximately 0.3% before and after CCH entered the market. Our reported prevalence of 0.3% is lower than recently published estimates, though it is close to a 0.5% prevalence reported in a previous population-based study [23]. While this is in part due to the younger age of our study population (average age, 57–60, annually), it likely reflects that PD is an underdiagnosed condition in population-based samples. In general, our findings are congruent with the accepted understanding that PD prevalence increases with age (as high as 0.48% in the 60–69 year-old age group). However, the prevalence in patients over 70 years old declined substantially. Whether this represents underdiagnosis or is directed by patient and provider desires requires further study.
Though we expected the prevalence of diagnosed PD to potentially increase after the approval of a new, minimally invasive treatment, this did not occur. However, we did note a gradual increase in prevalence of PD over the early years of our study. Though this may be related to slowly increasing awareness of PD in the community, it may also be true rise in incidence of PD over these years, mirroring the rise in incidence of associated comorbidities such as obesity, erectile dysfunction, and diabetes [15].
The annual percent of patients with PD receiving treatment increased significantly after CCH entered the market. This was associated with a significant increase in penile injections that offset overall declines in rates of surgical treatments for PD. Rates of PD treatment with AUA-recommended therapies has not been previously reported to our knowledge. However, it has been estimated that 59% to 72% of urologists initiate some therapy (including those outside AUA recommendations) for patients with PD, upon presentation [1617]. CCH availability was associated with a significant increase in annual cost of PD treatment per man. Though cost of PD treatment rose of the entire study period, the most pronounced increases were seen in the years after CCH became available. Unfortunately, we lack the required clinical data to comment on if this increase in cost is justified by improved patient outcomes. Future studies should assess if CCH is a cost-effective way to manage PD.
Corporal rupture is the most severe adverse event associated with CCH use. The proposed mechanism of action (enzymatic degradation of collagen) and occurrence of corporal rupture only in the CCH arm of the clinical trials validated concerns [8]. However, when considering all men with PD as a group, there was no change in the rate of corporal rupture before and after CCH became available. Though we did not find conclusive evidence that CCH significantly increases the risk of corporal rupture in men with PD, up to 34% of urologists using CCH have reported experience with a corporal rupture. It is possible that such events are not reliably captured using administrative claims data as non-specific codes (such as those for “penile hematoma”) may also have been used [9].
We acknowledge several limitations. The data used for our study does not include clinical outcomes of patients, so the efficacy of CCH in practice could not be assessed. Similarly, clinical data, such as severity of penile deformity/curvature, is not included. The number of corporal ruptures identified was small, limiting our ability to make definitive conclusions. Our study design cannot control for variations in diagnosis and coding behaviors between individual physicians. Though diverse, our study population is an insured population, and our findings may not be representative of uninsured patients, who many represent an undertreated group in general. However, our study uses of a large, well-maintained database that includes patients from various geographic and demographic strata in the United States. The coding data used for study inclusion are very specific to the conditions and treatments of interest. Finally, use of this type of database allows for evaluation of actual practice patterns and outcomes beyond the scope of clinical trials or institutional chart review.
This study investigates the effect of CCH availability on PD prevalence and treatment. While no change in PD prevalence occurred after CCH entered the market, a significant increase in the proportion of men with PD who received treatment, specifically with penile plaque injections, was found. In contrast, significantly less men underwent surgical repair for PD. At the same time, there was a significant increase in annual cost of PD treatment. Investigation into rates of corporal rupture found no change in men with PD as a whole after CCH entered the market.
References
1. Nehra A, Alterowitz R, Culkin DJ, Faraday MM, Hakim LS, Heidelbaugh JJ, et al. Peyronie's disease: AUA Guideline. J Urol. 2015; 194:745–753. PMID: 26066402.

2. Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. A population-based study of Peyronie's disease: prevalence and treatment patterns in the United States. Adv Urol. 2011; 2011:282503. PMID: 22110491.

3. Arafa M, Eid H, El-Badry A, Ezz-Eldine K, Shamloul R. The prevalence of Peyronie's disease in diabetic patients with erectile dysfunction. Int J Impot Res. 2006; 19:213–217. PMID: 16915304.

4. El-Sakka AI. Prevalence of Peyronie's disease among patients with erectile dysfunction. Eur Urol. 2006; 49:564–569. PMID: 16386353.

5. Nelson CJ, Mulhall JP. Psychological impact of Peyronie's disease: a review. J Sex Med. 2013; 10:653–660. PMID: 23153101.

6. Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: a long-term single-blind study. Urology. 1998; 51:620–626. PMID: 9586617.

7. Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009; 41:467–471. PMID: 19199072.

8. Gelbard M, Goldstein I, Hellstrom WJ, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013; 190:199–207. PMID: 23376148.

9. Yafi FA, Anaissie J, Zurawin J, Sikka SC, Hellstrom WJ. Results of SMSNA survey regarding complications following intralesional injection therapy with collagenase clostridium histolyticum for Peyronie's disease. J Sex Med. 2016; 13:684–689. PMID: 27045265.

10. Beilan JA, Wallen JJ, Baumgarten AS, Morgan KN, Parker JL, Carrion RE. Intralesional injection of collagenase clostridium histolyticum may increase the risk of late-onset penile fracture. Sex Med Rev. 2018; 6:272–278. PMID: 28923562.

11. Luo J, Seeger JD, Donneyong M, Gagne JJ, Avorn J, Kesselheim AS. Effect of generic competition on Atorvastatin prescribing and patients' out-of-pocket spending. JAMA Intern Med. 2016; 176:1317–1323. PMID: 27367749.

12. Maraka S, Mwangi R, McCoy RG, Yao X, Sangaralingham LR, Singh Ospina NM, et al. Thyroid hormone treatment among pregnant women with subclinical hypothyroidism: US national assessment. BMJ. 2017; 356:i6865. PMID: 28122781.

13. Desai RJ, Gagne JJ, Lii J, Liu J, Friedman S, Kim SC. Comparative risk of incident venous thromboembolism in patients with inflammatory bowel disease initiating tumour necrosis factor-α inhibitors or nonbiologic agents: a cohort study. CMAJ. 2017; 189:E1438–E1447. PMID: 29180383.

14. Pollack A. Injections to treat an embarrassing ailment win U.S. approval [Internet]. New York: The New York Times;c2017. cited 2017 Sep 22. Available from: https://www.nytimes.com/2013/12/07/business/fda-approves-treatment-forcurved-penis.html.
15. Weinberg AE, Eisenberg M, Patel CJ, Chertow GM, Leppert JT. Diabetes severity, metabolic syndrome, and the risk of erectile dysfunction. J Sex Med. 2013; 10:3102–3109. PMID: 24010555.

16. Sullivan J, Moskovic D, Nelson C, Levine L, Mulhall J. Peyronie's disease: urologist's knowledge base and practice patterns. Andrology. 2015; 3:260–264. PMID: 25331235.

17. LaRochelle JC, Levine LA. A survey of primary-care physicians and urologists regarding Peyronie's disease. J Sex Med. 2007; 4:1167–1173. PMID: 17627727.
Table 1
Demographic characteristics
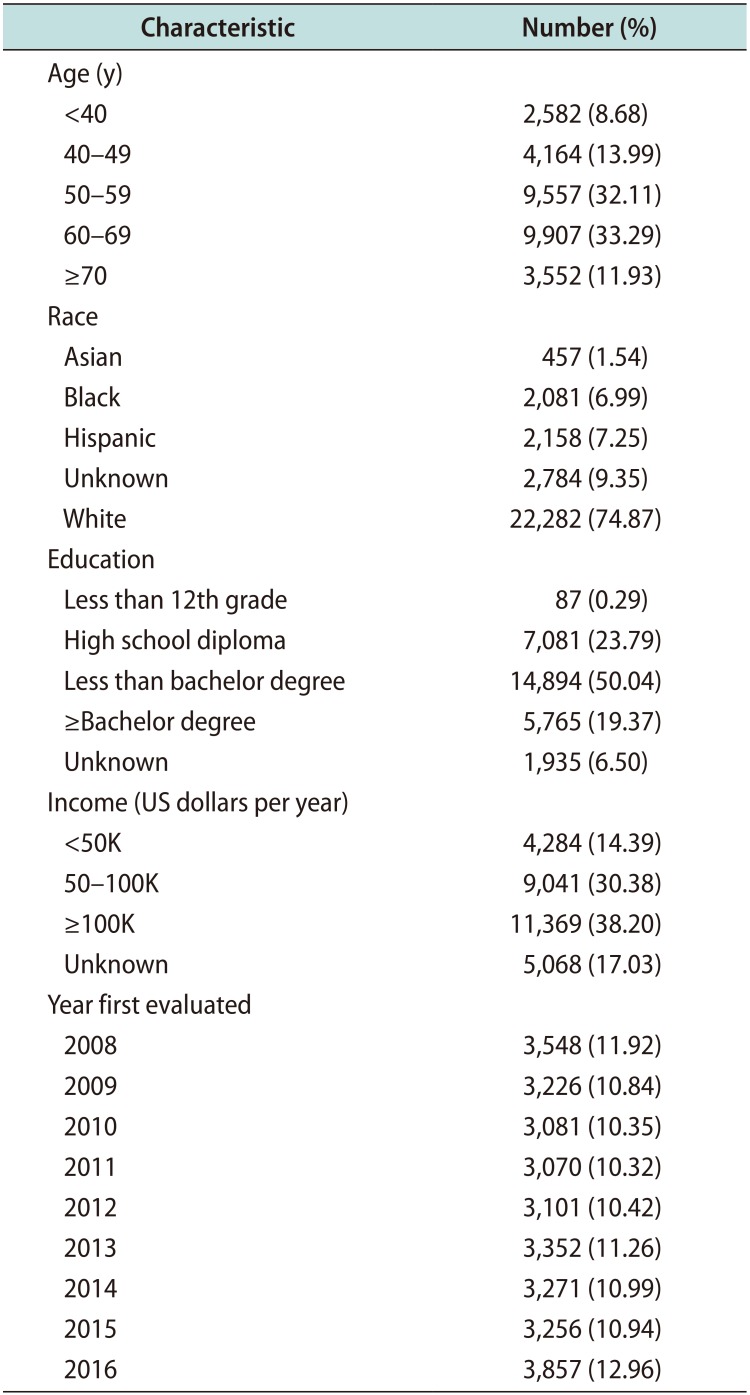
Table 2
Annual PD treatment and cost data




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download