This article has been
cited by other articles in ScienceCentral.
Abstract
Antiphospholipid antibodies may be produced in cases involving autoimmune diseases and can sometimes be caused by infections, such as Mycoplasma pneumoniae infection. However, antiphospholipid antibodies causing thrombosis associated with M. pneumoniae pneumonia in children have rarely been reported. We report a case of an 8-year-old boy with M. pneumoniae pneumonia with antiphospholipid antibodies, complicated by brachial artery thrombosis. He was found to have antiphospholipid antibodies and low protein S levels. The brachial artery thrombus was removed via thrombectomy. The titers of antiphospholipid antibodies turned normal within 5 months. This is a rare case of M. pneumoniae infection with brachial artery thrombosis associated with transient antiphospholipid antibodies.
초록
항인지질항체는 자가면역 질환에서 발생할 수 있고, 때로는 마이코플라즈마와 같은 감염 후에도 발생할 수 있다. 그러나 마이코플라즈마 폐렴 후 항인지질항체의 발현과 혈전의 발생은 매우 드물게 보고되어왔다. 본 8세 환아는 마이코플라즈마 폐렴 입원 치료 중 손가락의 변색 및 감각 저하가 발생하였고 혈액검사 결과 항인지질항체 양성으로 확인되었다. 이로 인해 상완동맥 혈전증이 발생하여 혈전제거술을 시행하여 제거하였다. 양성이었던 항인지질항체는 5개월 내 다시 정상화되었다. 마이코플라즈마 폐렴의 합병증은 피부 병변을 포함하여 체내 많은 장기에 다양한 증상을 보이지만, 그 중 항인지질항체의 발현으로 인한 혈전의 발생은 치명적이므로, 마이코플라즈마 폐렴의 치료에 있어 고려해야 할 합병증의 사례를 보고한다.
Keywords: Mycoplasma pneumoniae, Antiphospholipid antibodies, Thrombus
INTRODUCTION
Antiphospholipid antibodies may be produced in autoimmune diseases and these antibodies, including anticardiolipin antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulants, have been known to play a role in thrombophilic disorders affecting both venous and arterial circulation in antiphospholipid syndrome.
1)
Also, antiphospholipid antibodies have been reported in infection including viral and
Mycoplasma pneumoniae infection.
23) M. pneumoniae is a very common pathogen responsible for respiratory tract infections in children which has been reported in approximately 20–40% of community-acquired pneumonia cases in children.
4) Extrapulmonary manifestations of
M. pneumoniae are common, which includes arthritis, hepatitis, pericarditis, and hemolytic anemia.
45) However, thrombosis associated with transient antiphospholipid antibodies has rarely been reported in children with
M. pneumoniae infection.
Here, we report a case involving an 8-year-old boy with M. pneumoniae pneumonia who developed a brachial artery thrombus associated with transient antiphospholipid antibodies.
CASE
A previously healthy 8-year-old boy presented with a 1-week history of cough and a 3-day history of fever. After being treated with oral azithromycin for 2 days at a local clinic, he was transferred to a tertiary hospital owing to the worsening of his symptoms. Upon arrival, his body temperature was 39.3°C, he was tachypneic (36 breaths/min) with subcostal chest retraction, and his initial percutaneous oxygen saturation was 93%. On the day of admission, the C-reactive protein level increased to 6.37 mg/dL (reference range, 0.03–0.3 mg/dL), and
M. pneumoniae-specific immunoglobulin M (IgM) was detected in his serum: 2.4 EIU/mL (considered positive if >1.1 EIU/mL). Initial chest radiography showed lingular consolidation in the right upper lobe and bilateral lower lobe pneumonic infiltration (
Fig. 1A).
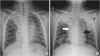
Fig. 1
(A) Initial CXR shows right upper lobar and lingular pneumonia, and bilateral lower lobe pneumonic infiltration. (B) Follow-up CXR on day 5 shows pneumomediastinum (white arrow), pneumopericardium (black arrow), and subcutaneous emphysema in the neck (white arrowhead) and the left chest wall (black arrowhead). Bilateral lower lobe bronchopneumonia is observed to have worsened.
Abbreviations: CXR, chest X-ray.
His symptoms worsened the following day, despite the administration of oral azithromycin and intravenous steroids. Oxygen therapy was initiated on the day 2 of hospitalization. On the day 5, he complained of neck and chest pain, and follow-up chest radiography revealed free air in the mediastinum, pericardial space, chest wall, and the neck (
Fig. 1B). Additionally, we noted collapse of both upper lobes and patchy consolidation in both lower lobes. Levofloxacin was added to cover for macrolide-resistant
M. pneumoniae. By the day 6, his respiratory symptoms improved, and follow-up chest radiography showed resolution of pneumonic infiltration and free air that was previously observed at the aforementioned locations.
However, on the day 8, the tip of his left index finger developed discoloration with the capillary refill time having increased to >4 seconds. Although the motor function in the index finger was intact, he showed diminished sensation in that finger. Doppler ultrasonography was performed to evaluate a possible thromboembolism; however, no radial or ulnar thrombosis was detected. Low-molecular-weight heparin (LMWH) was administered considering the possibility of the presence of a small embolus. On the day 11, the index finger showed no improvement, and administration of systemic urokinase was initiated. On the day 12, angiography of the left upper extremity revealed thrombotic obstruction of the left radial artery at the level of the first metacarpal head, interrupting the blood flow to the left thumb and index finger. The catheter tip was advanced to the distal left brachial artery, and urokinase was injected through the catheter. An intrabrachial arterial injection of urokinase was administered on 4 additional occasions on consecutive days.
Coagulation studies showed positive titers for anticardiolipin antibody IgM, anti-β2-glycoprotein I IgM, and lupus anticoagulant, and a low level of protein S (
Table 1). The cold agglutinin test was positive, and prothrombin time, partial thromboplastin time, and fibrinogen levels were within their respective reference ranges.
Table 1
Results of laboratory test for thrombophilia

|
Variables |
Results |
Reference range |
|
Anticardiolipin antibody IgG/IgM (U/mL) |
4.5/22.7 |
<10/<10 |
|
Anti-β2-glycoprotein I antibodies IgG/IgM (U/mL) |
0.4/15.3 |
<7/<7 |
|
Lupus anticoagulants |
Positive (1.35) |
0.8–1.19 |
|
Functional protein S activities (%) |
35 |
77–143 |
|
Functional protein C activities (%) |
117 |
70–130 |
|
Cold agglutinin test |
Positive (1:256) |
<1:16 |
|
Fibrinogen (mg/dL) |
237 |
166–408 |
|
D-dimer (µg/mL) |
>1,000 |
25.8–241 |
|
Prothrombin time (sec) |
14.7 |
9.6–12.1 |
|
Partial thromboplastin time (sec) |
39.1 |
25.3–41.1 |
Despite the local injection of urokinase, the brachial artery turned completely occluded, necessitating an emergency thrombectomy (
Fig. 2). On the day 17, emergency thrombectomy was successfully done. Postoperatively, the patient received nitroglycerin as vasodilator to maintain vessel dilatation and heparin for anticoagulation. He showed clinical improvement following thrombectomy. The color of his left index finger returned to normal, and his pulse was restored. Following the resolution of his clinical symptoms, he was discharged on the day 25 with the continuation of anticoagulation therapy including aspirin and clopidogrel.
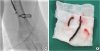
Fig. 2
(A) Angiography demonstrates acute thrombus formation in the left brachial artery. (B) The thrombus removed from the left brachial artery after thrombectomy.
He continued anticoagulation therapy over 4 months after thrombectomy and did not report any symptoms at follow-up. We observed that 5 months after discharge; he tested negative for the cold agglutinins and antiphospholipid antibodies, and accordingly, anticoagulation therapy was discontinued. This case report was approved by ethics committees of Korea University Medical Center to review the medical records (IRB No. 2018AS0074). Written informed consents were obtained from the parents of the patients prior all clinical procedures in accordance with standard clinical practice in our institute. And additional informed consent was obtained from parents on behalf of their child for whom identifying information is included in this case report.
DISCUSSION
We described the management of an 8-year-old boy with M. pneumoniae infection associated with brachial artery thrombosis. On evaluation for thrombosis in our patients, we found antiphospholipid antibodies and low protein S levels, which turned normal within 5 months after discharge. Follow-up laboratory results suggested that his prothrombotic state had not developed as a result of underlying thrombophilia but was attributable to the transient antiphospholipid antibodies induced by the M. pneumoniae infection.
There are 2 types of antiphospholipid antibodies, the autoimmune and infectious types. Antiphospholipid antibodies are commonly seen in autoimmune diseases and play a role in the pathogenesis of the thrombophilia in antiphospholipid syndrome.
6) Further, antiphospholipid antibodies are known to be potent mediators of acute inflammation in various infectious diseases. However, the presence of antiphospholipid antibodies does not always cause thrombosis in the infectious state.
2) To date, the pathophysiological role of antiphospholipid antibodies in thrombophilia has not been completely understood. It has been known that antiphospholipid antibodies alter hemostatic mechanisms via the inhibition of the fibrinolytic system and activation of the coagulation cascade secondary to the overproduction of tissue factors, which initiate the extrinsic pathway of coagulation. Moreover, antiphospholipid antibodies promote cellular activation of platelets, as well as the complement system, resulting in a hypercoagulable state.
67)
The pathogenic role of antiphospholipid antibodies in
M. pneumoniae infection remains unclear. Although increased levels in bacterial infections are not speculated to be associated with thrombotic episodes, a few cases of thrombotic events resulting in a cardiac thrombus, pulmonary embolism, and spleen infarct have been reported in children with
M. pneumoniae infections associated with transient antiphospholipid antibodies.
389) These children including our patient did not have any congenital factor predisposing them to thrombosis. There have been pathomechanisms suggesting that thrombosis is associated
M. pneumoniae infection. First,
M. pneumoniae infection may be a trigger for the induction of thrombosis.
M. pneumoniae causes a wide variety of extrapulmonary diseases including encephalitis, pericarditis, glomerulonephritis and arthritis. Autoimmune reactions have been suggested to be responsible for many of the extrapulmonary manifestations associated with
M. pneumoniae infection.
1011) These immune modulations may play a role in the induction of thrombosis. In previous pediatric cases, the patients experienced pulmonary complications in
M. pneumoniae pneumonia including pneumomediastinum, pleural effusion, and thrombus development more than 10 days after disease onset. Moreover, these patients were aged between 4 and 10 years, similar to the patient described in our report, suggesting that older children exhibit more extrapulmonary manifestations.
12) Another proposed mechanism is a direct invasion of
M. pneumoniae because the DNA of the pathogen was detected in cerebrospinal fluid of patients with stroke.
13) But this cannot explain thrombosis in others organ.
Anticoagulation is considered to be the standard therapy for the management of antiphospholipid syndrome.
14) The treatment of choice is unfractionated heparin, LMWH, or a pentasaccharide followed by the administration of a vitamin K antagonist.
1) The international normalized ratio should be maintained between 2.0 and 3.0 for an indefinite period in these patients. Patients who demonstrate only a single positive antiphospholipid antibody or do not present with a thrombophilic tendency or autoimmune disease may require only short-term anticoagulation therapy (3–6 months). Assessment for residual thrombosis must be performed prior to the cessation of anticoagulation therapy. The outcome of thrombotic events in
M. pneumoniae infection is usually favorable with the combination of antimicrobial treatment and anticoagulation therapy over 3–6 months.
15)
An evaluation for the presence of antiphospholipid antibodies should be considered in children who have an unusual thrombotic complication in M. pneumoniae pneumonia. Furthermore, association between antiphospholipid antibodies and M. pneumoniae infection requires additional studies for prevention of serious complication of M. pneumoniae infection.
ACKNOWLEDGEMENT
All authors wish to thank the staff at the Pediatrics Department of the Korea University Ansan Hospital for their help in this case.
References
1. Tripodi A, de Groot PG, Pengo V. Antiphospholipid syndrome: laboratory detection, mechanisms of action and treatment. J Intern Med. 2011; 270:110–122.


3. Bakshi M, Khemani C, Vishwanathan V, Anand RK, Khubchandani RP. Mycoplasma pneumonia with antiphospholipid antibodies and a cardiac thrombus. Lupus. 2006; 15:105–106.


4. Defilippi A, Silvestri M, Tacchella A, Giacchino R, Melioli G, Di Marco E, et al. Epidemiology and clinical features of
Mycoplasma pneumoniae infection in children. Respir Med. 2008; 102:1762–1768.


6. Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet. 2010; 376:1498–1509.


7. Comarmond C, Cacoub P. Antiphospholipid syndrome: from pathogenesis to novel immunomodulatory therapies. Autoimmun Rev. 2013; 12:752–757.


8. Witmer CM, Steenhoff AP, Shah SS, Raffini LJ.
Mycoplasma pneumoniae, splenic infarct, and transient antiphospholipid antibodies: a new association? Pediatrics. 2007; 119:e292–e295.
9. Graw-Panzer KD, Verma S, Rao S, Miller ST, Lee H. Venous thrombosis and pulmonary embolism in a child with pneumonia due to
Mycoplasma pneumoniae
. J Natl Med Assoc. 2009; 101:956–958.


10. Waites KB, Talkington DF.
Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004; 17:697–728.



11. Narita M. Pathogenesis of extrapulmonary manifestations of
Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010; 16:162–169.


12. Youn YS, Lee KY, Hwang JY, Rhim JW, Kang JH, Lee JS, et al. Difference of clinical features in childhood
Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010; 10:48.

13. Padovan CS, Pfister HW, Bense S, Fingerle V, Abele-Horn M. Detection of Mycoplasma pneumoniae DNA in cerebrospinal fluid of a patient with M. pneumoniae infection-“associated” stroke. Clin Infect Dis. 2001; 33:E119–21.
14. Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995; 332:993–997.


15. Flateau C, Asfalou I, Deman AL, Ficko C, Andriamanantena D, Fontan E, et al. Aortic thrombus and multiple embolisms during a
Mycoplasma pneumoniae infection. Infection. 2013; 41:867–873.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download