1. Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration--communication of cells. Clin Oral Implants Res. 2012; 23:1127–1135.

2. Tomisa AP, Launey ME, Lee JS, Mankani MH, Wegst UG, Saiz E. Nanotechnology approaches to improve dental implants. Int J Oral Maxillofac Implants. 2011; 26:Suppl. 25–44.
3. Aljateeli M, Wang HL. Implant microdesigns and their impact on osseointegration. Implant Dent. 2013; 22:127–132.

4. Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol. 2010; 47:1–4.

5. Ong JL, Chan DC. Hydroxyapatite and their use as coatings in dental implants: a review. Crit Rev Biomed Eng. 2000; 28:667–707.


6. Whitehead RY, Lucas LC, Lacefield WR. The effect of dissolution on plasma sprayed hydroxylapatite coatings on titanium. Clin Mater. 1993; 12:31–39.


7. Wheeler SL. Eight-year clinical retrospective study of titanium plasma-sprayed and hydroxyapatite-coated cylinder implants. Int J Oral Maxillofac Implants. 1996; 11:340–350.


8. Thierer T, Davliakos JP, Keith JD Jr, Sanders JJ, Tarnow DP, Rivers JA. Five-year prospective clinical evaluation of highly crystalline HA MP-1-coated dental implants. J Oral Implantol. 2008; 34:39–46.


9. Artzi Z, Carmeli G, Kozlovsky A. A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5-10 years in function. Clin Oral Implants Res. 2006; 17:85–93.


10. Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006; 76:323–334.


11. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–533.


12. Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A. The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res A. 2007; 82:658–668.


13. Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2010; 93:289–296.


14. Lang NP, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Bosshardt DD. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res. 2011; 22:349–356.


15. Calciolari E, Mardas N, Dereka X, Anagnostopoulos AK, Tsangaris GT, Donos N. Protein expression during early stages of bone regeneration under hydrophobic and hydrophilic titanium domes. A pilot study. J Periodontal Res. 2018; 53:174–187.


16. Matsuo K, Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008; 473:201–209.


17. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8:e1000412.

18. Vlacic-Zischke J, Hamlet SM, Friis T, Tonetti MS, Ivanovski S. The influence of surface microroughness and hydrophilicity of titanium on the up-regulation of TGFβ/BMP signalling in osteoblasts. Biomaterials. 2011; 32:665–671.


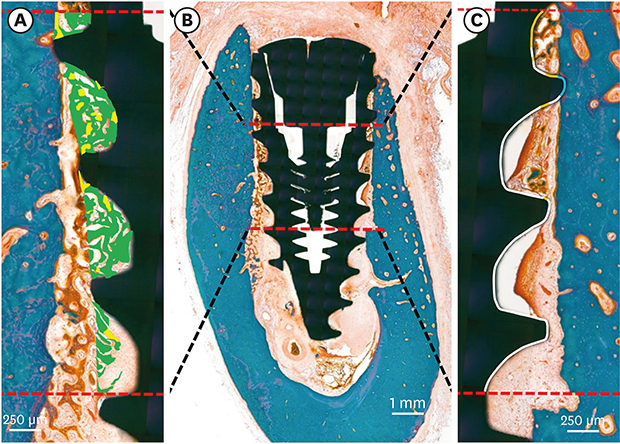
19. Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982; 11:318–326.


21. Sato M, Aslani A, Sambito MA, Kalkhoran NM, Slamovich EB, Webster TJ. Nanocrystalline hydroxyapatite/titania coatings on titanium improves osteoblast adhesion. J Biomed Mater Res A. 2008; 84:265–272.


22. Sohn SH, Jun HK, Kim CS, Kim KN, Chung SM, Shin SW, et al. Biological responses in osteoblast-like cell line according to thin layer hydroxyapatite coatings on anodized titanium. J Oral Rehabil. 2006; 33:898–911.


23. Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, et al. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int J Oral Maxillofac Implants. 2007; 22:755–760.

24. Stadlinger B, Lode AT, Eckelt U, Range U, Schlottig F, Hefti T, et al. Surface-conditioned dental implants: an animal study on bone formation. J Clin Periodontol. 2009; 36:882–891.


25. Schenk RK, Buser D. Osseointegration: a reality. Periodontol 2000. 1998; 17:22–35.


26. Futami T, Fujii N, Ohnishi H, Taguchi N, Kusakari H, Ohshima H, et al. Tissue response to titanium implants in the rat maxilla: ultrastructural and histochemical observations of the bone-titanium interface. J Periodontol. 2000; 71:287–298.


27. Haider R, Watzek G, Plenk H. Effects of drill cooling and bone structure on IMZ implant fixation. Int J Oral Maxillofac Implants. 1993; 8:83–91.

28. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.


29. Ong JL, Carnes DL, Bessho K. Evaluation of titanium plasma-sprayed and plasma-sprayed hydroxyapatite implants
in vivo
. Biomaterials. 2004; 25:4601–4606.


30. Park YS, Yi KY, Lee IS, Han CH, Jung YC. The effects of ion beam-assisted deposition of hydroxyapatite on the grit-blasted surface of endosseous implants in rabbit tibiae. Int J Oral Maxillofac Implants. 2005; 20:31–38.

31. Orsini G, Piattelli M, Scarano A, Petrone G, Kenealy J, Piattelli A, et al. Randomized, controlled histologic and histomorphometric evaluation of implants with nanometer-scale calcium phosphate added to the dual acid-etched surface in the human posterior maxilla. J Periodontol. 2007; 78:209–218.


32. Mendes VC, Moineddin R, Davies JE. The effect of discrete calcium phosphate nanocrystals on bone-bonding to titanium surfaces. Biomaterials. 2007; 28:4748–4755.










 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download