Abstract
Endoplasmic reticulum (ER) stress is mediated by disturbance of Ca2+ homeostasis. The store-operated calcium (SOC) channel is the primary Ca2+ channel in non-excitable cells, but its participation in agent-induced ER stress is not clear. In this study, the effects of tunicamycin on Ca2+ influx in human umbilical vein endothelial cells (HUVECs) were observed with the fluorescent probe Fluo-4 AM. The effect of tunicamycin on the expression of the unfolded protein response (UPR)-related proteins BiP and CHOP was assayed by western blotting with or without inhibition of Orai1. Tunicamycin induced endothelial dysfunction by activating ER stress. Orai1 expression and the influx of extracellular Ca2+ in HUVECs were both upregulated during ER stress. The SOC channel inhibitor SKF96365 reversed tunicamycin-induced endothelial cell dysfunction by inhibiting ER stress. Regulation of tunicamycin-induced ER stress by Orai1 indicates that modification of Orai1 activity may have therapeutic value for conditions with ER stress-induced endothelial dysfunction.
Endothelial dysfunction is a cause of cardiovascular diseases [12]. Endoplasmic reticulum (ER) stress regulates apoptosis and inflammation in endothelial cells [34], and contributes to obesity, heart disease, atherosclerosis, diabetes [56]. The ER is the site of protein synthesis, folding, and transport. Misfolding, unfolding, and accumulation of proteins in the ER provoke the unfolded protein response (UPR)-ER stress. The UPR is mediated by three transmembrane ER sensors, inositol-requiring 1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). The stress sensors are inactivated in resting cells by binding to immunoglobulin heavy chain-binding protein (BiP), an ER resident chaperone molecule. When the UPR is initiated, BiP is released from these complexes and binds to unfolded or misfolded polypeptide chains, which leads to the activation of ER stress sensors [7]. XBP1s is produced by the activation of IRE1α, and it acts as a potent transcription factor to regulate the expression of UPR-related genes. When PERK releases from BiP, activation of PERK leads to the translation of the transcription factor ATF4, follows with the expression of the proapoptotic transcription factor C/EBP-homologous protein (CHOP), which encourages ROS and apoptosis in ER stress [8].
ER stress is related to disturbance of Ca2+ homeostasis [9], and there is evidence that it is mediated by calcium overload. Thapsigargin, an inhibitor of sarco/endoplasmic reticulum Ca2+-ATPase, and tunicamycin-an inhibitor of protein glycosylation, both induce UPR by disrupting ER Ca2+ homeostasis. Store-operated calcium channel (SOC) is the main regulator of Ca2+ homeostasis in non-excitable cells, such as endothelial cells [10]. Orai1 and STIM1 were the molecular basis of store-operated Ca2+ entry (SOCE) in endothelial cells and could mediate the proliferation of the cells [11]. However, whether the two key components of SOC channel Orai1 and STIM1 participated in the ER stress induced endothelial dysfunction is not clear. This study investigated the role of Orai1-mediated Ca2+ entry in ER stress-mediated endothelial dysfunction.
Human umbilical vein endothelial cells (HUVECs) were isolated from the umbilical vein and cultured as previously described [12]. In brief, HUVECs were digested with 0.125% trypsin containing 0.01% EDTA, then the cells were cultured in “complete M199 medium” which containing 20% fetal calf serum, 100 U/ml penicillin, and 100 µg/ml streptomycin, 25 U/ml heparin, 2 mmol/L L-glutamine and 5 ng/ml recombinant human endothelial cell growth factor β at 37℃, 5% CO2 atmosphere. After reaching 80% confluence, cells were subcultured with 0.125% trypsin with 0.01% EDTA.
Cell viability was measured by a Cell Counting Assay Kit-8 (CCK-8; Dojindo Molecular Technologies, MD, Japan) following the manufacturer's instructions. Briefly, HUVECs were seeded in 96-well plates at a density of 1×104 cell/ml and cultured for 24 h. Cells were treated with 0.25, 0.5, 1, or 2 µg/ml tunicamycin for 24 h before adding 10 µl CCK-8 to each well. After 2 h at 37℃, absorbance at 450 nm was measured with a Multiskan GO Microplate Spectrophotometer (Thermo, USA).
HUVECs were rinsed with ice-cold PBS, homogenized in a blender and lysed with RIPA lysis buffer containing a protease inhibitor cocktail (Merck, USA). The protein concentration was determined with a Bicinchoninic Acid kit (Thermo Scientific, USA). Proteins was separated by SDS-PAGE and transferred to PVDF membranes (Millipore, Billerica, MA, USA). After incubation with 5% non-fat dry milk diluted with TBST at room temperature for 1 h, membranes were incubated with primary antibody against eNOS (1:1000, Cell Signaling Technology, USA), Orai1 (1:1000, Alomone, Israel), BiP (1:1000, Cell Signaling Technology, USA), CHOP (1:1000, Cell Signaling Technology, USA), XBP1s (1:1000, Cell Signaling Technology, USA) at 4℃ overnight. They were then incubated for 1 h with appropriate HRP-conjugated secondary antibodies (Cell Signaling Technology, USA) at room temperature. GAPDH was used as the loading control. Bands were detected with Pierce enhanced chemiluminescence (ECL) western blotting substrate (Thermo Scientific) and quantified with Image J software (National Institutes of Health, USA).
Intracellular Ca2+ concentration was assayed by the Fluo-4 AM calcium probe (Invitrogen, USA). Briefly, the HUVECs were incubated in Hank's balanced salt solution (mmol/L: NaCl 138, KCl 5.3, KH2PO4 0.4, MgCl2·6H2O 0.5, MgSO4·7H2O 0.4, CaCl2 1.26, NaHCO3 25, Na2HPO4 0.34, D-glucose 5.56, pH 7.4) containing 5 µmol/L Fluo-4 AM for 30 min at 37℃. Cells were washed three times with the Ca2+-free HBSS and incubated for 30 minutes to allow complete de-esterification of intracellular AM esters. The intracellular Ca2+ concentration was measured at an excitation wavelength of 485 nm and emission wavelength of 520 nm with inverted confocal laser scanning microscope (SP5-FCS, Leica, Germany). Intracellular calcium concentration was reported as the % change of fluorescence intensity from baseline. [(F–F0)/F0×100], where F0 was the resting Fluo-4 fluorescence.
HUVECs were transfected with short hairpin (sh)RNA adenovirus against Orai1 genes (Shanghai GeneChem). A scrambled shRNA adenovirus was used as a negative control. HUVECs were seeded at 2×105 cells/ml in 6-well plates. Before transfection, the culture medium was replaced with 1 ml complete media, and Ad-Orai1 shRNA was added to the cells for 6 h at 37℃. The transfected cells were cultured in 2 ml of normal culture medium for an additional 48 h at 37℃.
Tunicamycin, SKF96365 and tauroursodeoxycholic acid (TUDCA) were purchased from Sigma (St. Louis, MO). Stock solutions were prepared by dissolving in dimethyl sulfoxide.
Data are represented as means ± S.E.M. Statistical significance was determined with the unpaired two-tailed Student t-test or one-way ANOVA followed by the Bonferroni multiple comparison post hoc test with calculation of 95% confidence intervals. Statistical significance was accepted when p < 0.05.
Tunicamycin inhibited the proliferation and promoted the apoptosis of endothelial cells, and significantly decreased endothelial nitric oxide synthase (eNOS) expression. It also increased the expression of the UPR-related proteins BiP and CHOP in human umbilical vein cells (HUVECs). BiP expression increased to 1.72 ± 0.19 at 0.25 µg/ml, 2.09 ± 0.07 at 0.5 µg/ml, 2.03 ± 0.07 at 1 µg/ml, and 1.79 ± 0.10 at 2 µg/ml tunicamycin, compared with the controls. CHOP was weakly expressed in the control and DMSO-treated groups and was significantly enhanced by tunicamycin (Fig. 1). These results suggested that ER stress induced by tunicamycin was involved in endothelial dysfunction.
The effect of tunicamycin on Ca2+ concentration was assayed in endothelial cells after depletion of ER calcium stores by 2 µM thapsigargin, which secondarily activated the SOC channel-mediated Ca2+ influx. Incubation with tunicamycin significantly increased SOCE (Fig. 2).
Disturbed Ca2+ homeostasis was associated with ER stress, and the SOC channel was the primary pathway of calcium influx in endothelial cells. As Orai1 is the major component of the SOC channel, the effect of tunicamycin on Orai1 expression in HUVECs was assayed. The relative expression of Orai1 was 1.82 ± 0.17 at 0.25 µg/ml, 2.08 ± 0.22 at 0.5 µg/ml, 2.26 ± 0.29 at 1 µg/ml, and 2.59 ± 0.24 at 2 µg/ml tunicamycin, compared with the controls (Fig. 3). The ER stress inhibitor TUDCA decreased Orai1 expression induced by tunicamycin (Supplementary Fig. 1). These results showed that Orai1 may be involved in tunicamycin-induced ER stress.
To clarify the role of Orai1 in ER stress, HUVECs were preincubated with a calcium channel inhibitor (SKF96365) or adenovirus-Orai1 siRNA before tunicamycin treatment. SKF96365 significantly decreased BiP and CHOP expression from 2.10 ± 0.07 to 0.95 ± 0.25 and 7.81 ± 0.51 to 2.55 ± 0.65 relative to treatment with tunicamycin alone. SKF96365 also significantly inhibited the downregulation of eNOS induced by tunicamycin (Fig. 4). SKF96365 only blocked capacitive Ca2+ entry, which did not affect Orai1 expression (Supplementary Fig. 2). Knockdown of Orai1 also reversed BiP, XBP1s and eNOS expression induced by tunicamycin (Fig. 5), consistent with reduced tunicamycin-induced ER stress and protection of endothelial function. Orai1 may thus have mediated endothelial dysfunction during ER stress.
This study found that tunicamycin promoted endothelial dysfunction by inducing ER stress. It enhanced Orai1 expression and SOC channel-mediated Ca2+ entry in HUVECs. Inhibition of Orai1 by SKF96365 or knockdown of Orai1 by adenovirus-Orai1 siRNA reversed the expression of BiP and CHOP proteins and improved endothelial dysfunction induced by tunicamycin.
Endothelial dysfunction characterizes cardiovascular diseases like atherosclerosis, hypertension, and diabetes mellitus, and ER stress is involved in the pathogenesis cardiovascular diseases. Tunicamycin, an inhibitor of protein glycosylation, was used experimentally as an ER stressor to induce endothelial dysfunction in cultured HUVECs. Calcium storage in the ER is one of the main ER functions, and Ca2+ depletion activates SOCE which increases intracellular Ca2+ influx. SOCE directly or indirectly regulates the Ca2+-dependent UPR [1314]. Tunicamycin has been shown to increase intracellular Ca2+ concentration in lymphoid and smooth muscle cells by SOCE [1516], but the specific calcium channel was not determined.
There are two types of calcium channels in endothelial cells, L-type channels (Cav1.2) and SOC channels, but as endothelial cells are non-excitable, Cav1.2 channels are rare, the majority are SOC channels. Orai1 and STIM1 are both components of SOC channel [1718], which are expressed in endothelial cells and involved in cell proliferation [11]. Orai1 and STIM1 have been reported to mediate histamine-induced inflammation in HUVECs [19], and Orai1 was found to be involved in VEGF-induced migration of endothelial cells and endothelial tube formation [20]. This study provided evidence that Orai1-mediated Ca2+ entry contributed to ER stress induced in HUVECs by tunicamycin.
Pathological stimuli including oxidative stress, inflammation and hypoxia can induce the UPR, and ER stress may contribute to endothelial dysfunction [42122]. Activation of ER stress has been reported in cardiovascular diseases including arteriosclerosis, ischemia/reperfusion injury, and heart failure [232425]. The maintenance of Ca2+ homeostasis depends on Ca2+ transporters, channels, and binding/buffering proteins. ER stress is a consequence of disturbed calcium homeostasis [9], and SOCE is active in Ca2+ transport during ER stress. In dopaminergic neurons, transient receptor potential channel 1 (TRPC1) was found to regulate Ca2+ homeostasis and inhibit UPR, which contributed to neuronal survival [26]. In hepatic cells, Orai1 and SOCE were reported to mediate usnic acid toxicity by enhancing ER stress [14], but the calcium channel regulating ER stress in endothelial cells remains unclear. Both Orai1 and Orai1-mediated SOCE were increased by tunicamycin, and pharmacological inhibition of SOCE by SKF96365 or silencing of Orai1 decreased the expression of the UPR-related proteins BiP, CHOP and XBP1s. eNOS is a Ca2+-dependent enzyme that regulates vascular tone and function. Decrease in eNOS expression and activity leads to endothelial dysfunction. Previous study reported that SOCE inhibited eNOS activation and availability by activating Calpain [27]. In the present study, we found out that Orai1-mediated SOCE was involved in tunicamycin-induced decrease of eNOS expression, which may attribute to the induction of ER stress. Celine et al reported that CHOP-10, an UPR-related protein, transcriptionally repressed eNOS by modulating the activity of eNOS gene promoter [28]. However, Ziomek et al. [16] found that tunicamycin induced a significant increase of intracellular Ca2+ in vascular smooth muscle cells, but it was dependent on the direct permeability of the plasma membrane, ER, and sarcoplasmic reticulum to calcium, not on the activation of calcium channels. This difference may be a consequence to the different cell types that were used. Further study is needed to confirm the role of Orai1 in the tunicamycinmediated Ca2+ influx in ECs. In summary, Orai1 participated in the development of ER stress in cultured HUVECs. If it is determined that Orai1 mediates endothelial dysfunction in diabetes via ER stress, it may prove to be a potential therapeutic target for vascular complications of diabetes.
Figures and Tables
Fig. 1
Tunicamycin induced endothelial dysfunction by ER stress.
HUVECs were treated with 0.25, 0.5, 1, 2 µg/ml tunicamycin for 24 h. Cell viability was assayed with Cell Counting Assay Kit-8 (A). Expression of eNOS (B), BiP (C) and CHOP (D) was determined by western blotting. GAPDH was used as an internal control. *p < 0.05 vs. control; n = 4.
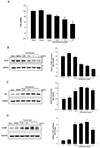
Fig. 2
Alteration of intracellular Ca2+ concentration in HUVECs induced by tunicamycin.
HUVECs were treated with 1 µg/ml tunicamycin for 24 h. Cells were incubated in a Ca2+-free buffer containing 2 µM thapsigargin for 8 min, and Ca2+ influx was induced during the Ca2+ loading period (left panel). Ca2+ influx was enhanced by tunicamycin (right panel). **p < 0.01 vs. control; n = 60 cells.

Fig. 3
Orai1 expression was upregulated by tunicamycin in HUVECs.
HUVECs were treated with tunicamycin (0.25, 0.5, 1, 2 µg/ml respectively). Twenty-four hours later, the expression of Orai1 was determined by western blotting. *p < 0.05 vs. control; n = 4.

Fig. 4
SOC channel inhibitor SKF-96365 reduced tunicamycin-induced ER stress to protect endothelial function.
HUVECs were preincubated with 1.25, 2.5, 5 µM SKF96365 before treatment with 1 µg/ml tunicamycin. BiP (A), CHOP (B), and eNOS (C) expression were determined by western blotting. *p < 0.05 vs. control; #p < 0.05 vs. tunicamycin treatment only, n = 4.
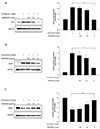
Fig. 5
Effect of Orai1 siRNA on tunicamycin-induced eNOS and UPR markers expression.
HUVECs were preincubated with 50, 100, or 200 MOI adenovirus-Orai1siRNA for 24 h; 1 µg/ml tunicamycin was added for another 24 h. (A) eNOS, UPR markers and Orai1 expression were assayed by western blotting. (B-E) Densitometric analysis of the protein levels of eNOS, BiP, Xbp1-s and Orai1 respectively, GAPDH was used as the internal control. *p < 0.05, **p < 0.01 vs. control; #p < 0.05, ##p < 0.01 vs. tunicamycin treatment only, n = 4.

ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (No 81302779, No 81273516, No 81470440, No 81370295), and Guangdong Provincial Medical Science Foundation (No A2015229).
Notes
Author contributions H.Y. and C.Y.D. conceived and designed the experiments. H.Y. and Y.M.X. performed the cell-based assay experiments. S.J.K. performed the Ca2+ measurement. M.Z.Z., J.H.C., L.L., Z.X.S., Q.X.L., X.H.L., M.Y. and H.Z. coordinated the study. H.Y., F.R. and C.Y.D. analyzed the data and wrote the manuscript.
References
1. Xu J, Zou MH. Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009; 120:1266–1286.



2. Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003; 23:168–175.

3. Lu Y, Qian L, Zhang Q, Chen B, Gui L, Huang D, Chen G, Chen L. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life Sci. 2013; 92:1165–1173.


4. Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006; 26:2490–2496.


5. Lenna S, Han R, Trojanowska M. Endoplasmic reticulum stress and endothelial dysfunction. IUBMB Life. 2014; 66:530–537.



6. Ozcan L, Tabas I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu Rev Med. 2012; 63:317–328.



7. Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem. 2012; 81:767–793.



8. Groenendyk J, Agellon LB, Michalak M. Coping with endoplasmic reticulum stress in the cardiovascular system. Annu Rev Physiol. 2013; 75:49–67.


9. Krebs J, Agellon LB, Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem Biophys Res Commun. 2015; 460:114–121.

10. Nilius B, Viana F, Droogmans G. Ion channels in vascular endothelium. Annu Rev Physiol. 1997; 59:145–170.


11. Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 mediate CRAC currents and storeoperated calcium entry important for endothelial cell proliferation. Circ Res. 2008; 103:1289–1299.



12. Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007; 2:481–485.


13. Groenendyk J, Peng Z, Dudek E, Fan X, Mizianty MJ, Dufey E, Urra H, Sepulveda D, Rojas-Rivera D, Lim Y, Kim DH, Baretta K, Srikanth S, Gwack Y, Ahnn J, Kaufman RJ, Lee SK, Hetz C, Kurgan L, Michalak M. Interplay between the oxidoreductase PDIA6 and microRNA-322 controls the response to disrupted endoplasmic reticulum calcium homeostasis. Sci Signal. 2014; 7:ra54.

14. Chen S, Zhang Z, Wu Y, Shi Q, Yan H, Mei N, Tolleson WH, Guo L. Endoplasmic reticulum stress and store-operated calcium entry contribute to usnic acid-induced toxicity in hepatic cells. Toxicol Sci. 2015; 146:116–126.



15. Czyź A, Brutkowski W, Fronk J, Duszyński J, Zabłocki K. Tunicamycin desensitizes store-operated Ca2+ entry to ATP and mitochondrial potential. Biochem Biophys Res Commun. 2009; 381:176–180.

16. Ziomek G, Cheraghi Zanjani P, Arman D, van Breemen C, Esfandiarei M. Calcium regulation in aortic smooth muscle cells during the initial phase of tunicamycin-induced endo/sarcoplasmic reticulum stress. Eur J Pharmacol. 2014; 735:86–96.


17. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005; 15:1235–1241.
18. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006; 443:230–233.


19. Zhou MH, Zheng H, Si H, Jin Y, Peng JM, He L, Zhou Y, Muñoz-Garay C, Zawieja DC, Kuo L, Peng X, Zhang SL. Stromal interaction molecule 1 (STIM1) and Orai1 mediate histamine-evoked calcium entry and nuclear factor of activated T-cells (NFAT) signaling in human umbilical vein endothelial cells. J Biol Chem. 2014; 289:29446–29456.



20. Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, Majeed Y, Tumova S, Seymour VA, Taylor H, Stacey M, O'Regan D, Foster R, Porter KE, Kearney MT, Beech DJ. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res. 2011; 108:1190–1198.


21. Huang H, Jing G, Wang JJ, Sheibani N, Zhang SX. ATF4 is a novel regulator of MCP-1 in microvascular endothelial cells. J Inflamm (Lond). 2015; 12:31.



22. Cheang WS, Tian XY, Wong WT, Lau CW, Lee SS, Chen ZY, Yao X, Wang N, Huang Y. Metformin protects endothelial function in diet-induced obese mice by inhibition of endoplasmic reticulum stress through 5′ adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor δ pathway. Arterioscler Thromb Vasc Biol. 2014; 34:830–836.


23. Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003; 5:781–792.


24. Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005; 115:2656–2664.



25. Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004; 110:705–712.

26. Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest. 2012; 122:1354–1367.



27. Stalker TJ, Gong Y, Scalia R. The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes. 2005; 54:1132–1140.


28. Loinard C, Zouggari Y, Rueda P, Ramkhelawon B, Cochain C, Vilar J, Récalde A, Richart A, Charue D, Duriez M, Mori M, Arenzana-Seisdedos F, Lévy BI, Heymes C, Silvestre JS. C/EBP homologous protein-10 (CHOP-10) limits postnatal neovascularization through control of endothelial nitric oxide synthase gene expression. Circulation. 2012; 125:1014–1026.


SUPPLEMENTARY MATERIALS
Supplementary data including two figures can be found with this article online at http://pdf.medrang.co.kr/paper/pdf/Kjpp/Kjpp2019-23-02-01-s001.pdf.
Supplementary Fig. 1
Effect of the ER stress inhibitor TUDCA on tunicamycin-induced Orai1 expression. HUVECs were preincubated with 0.25, 0.5, 1 mM TUDCA before treatment with 1 µg/ml tunicamycin for 24 h. Orai1 expression was assayed by western blotting. *p < 0.05 vs. control; #p < 0.05 vs. tunicamycin treatment only, n = 4.
Supplementary Fig. 2
Effect of the SOCE inhibitor SKF96365 on tunicamycin-induced Orai1 and STIM1 expression. HUVECs were preincubated with 1.25, 2.5, or 5 µM SKF96365 before treatment with 1 µg/ml tunicamycin for 24 h. Orai1 and STIM1 expression were assayed by western blotting. *p < 0.05 vs. control, n = 4.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download