2. Iakovides J, Delides GS. Carcinosarcomas of the skin--report of two cases. Arch Geschwulstforsch. 1988; 58(6):461–464.

3. Zidar N, Gale N. Carcinosarcoma and spindle cell carcinoma--monoclonal neoplasms undergoing epithelial-mesenchymal transition. Virchows Arch. 2015; 466(3):357–358.


4. Gungorduk K, Ozdemir A, Ertas IE, Gokcu M, Telli E, Oge T, et al. Adjuvant treatment modalities, prognostic predictors and outcomes of uterine carcinosarcomas. Cancer Res Treat. 2015; 47(2):282–289.

5. Au JT, Sugiyama G, Wang H, Nicastri A, Lee D, Ko W, et al. Carcinosarcoma of the oesophagus - a rare mixed type of tumor. J Surg Case Rep. 2010; 2010(7):7.

7. Dawson EK. Carcino-sarcoma of the skin. J R Coll Surg Edinb. 1972; 17(4):243–246.

8. Calonje E, Brenn T, Lazar A, Mckee PH. Tumors of the surface epithelium. In : Calonje E, Brenn T, Lazar A, Mckee PH, editors. McKee's Pathology of the Skin. 4th ed. Oxford: Elsevier Saunders;2012. p. 1139–1140.
9. Tran TA, Muller S, Chaudahri PJ, Carlson JA. Cutaneous carcinosarcoma: adnexal vs. epidermal types define high- and low-risk tumors. Results of a meta-analysis. J Cutan Pathol. 2005; 32(1):2–11.


10. Patel NK, McKee PH, Smith NP, Fletcher CD. Primary metaplastic carcinoma (carcinosarcoma) of the skin. A clinicopathologic study of four cases and review of the literature. Am J Dermatopathol. 1997; 19(4):363–372.

11. Bigby SM, Charlton A, Miller MV, Zwi LJ, Oliver GF. Biphasic sarcomatoid basal cell carcinoma (carcinosarcoma): four cases with immunohistochemistry and review of the literature. J Cutan Pathol. 2005; 32(2):141–147.


13. Clark JJ, Bowen AR, Bowen GM, Hyngstrom JR, Hadley ML, Duffy K, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017; 44(1):34–44.


14. Syme-Grant J, Syme-Grant NJ, Motta L, Stevenson JH, Evans AT. Are primary cutaneous carcinisarcomas underdiagnosed? Five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006; 59(12):1402–1408.


15. Noh SH, Chae JK, Park SH, Kim EJ, Park K. Primary cutaneous carcinosarcoma composed of squamous cell carcinoma and epithelioid sarcoma. Korean J Dermatol. 2016; 54(2):145–148.
16. Song KH, Kim DW, Kim JI, Hwang SR, Park J, Noh SK, et al. Primary cutaneous carcinosarcoma of the scalp. Korean J Dermatol. 2013; 51(11):928–930.
17. Rhee CH, Song KH, Cho YS, Nam KH, Yun SK, Kim HU, et al. A case of cutaneous carcinosarcoma. Korean J Dermatol. 2010; 48(6):521–524.
18. Rose RF, Merchant W, Stables GI, Lyon CL, Platt A. Basal cell carcinoma with a sarcomatous component (carcinosarcoma): a series of 5 cases and a review of the literature. J Am Acad Dermatol. 2008; 59(4):627–632.


19. Loh TL, Tomlinson J, Chin R, Eslick GD. Cutaneous carcinosarcoma with metastasis to the parotid gland. Case Rep Otolaryngol. 2014; 2014:173235.

20. Dadzie OE, Mahalingam M. Pearls and Pitfalls in Neoplastic Dermatopathology. Cambridge: Cambridge Medicine;2016.
21. Thompson L, Chang B, Barsky SH. Monoclonal origins of malignant mixed tumors (carcinosarcomas). Evidence for a divergent histogenesis. Am J Surg Pathol. 1996; 20(3):277–285.

22. Harrist TJ, Hassell LA, Bronstein BR, Mihm MC Jr. Follow-up of a previously reported carcinosarcoma of the skin. J Cutan Pathol. 1983; 10(5):359–360.


23. Velazquez EF, Melamed J, Barreto JE, Aguero F, Cubilla AL. Sarcomatoid carcinoma of the penis: a clinicopathologic study of 15 cases. Am J Surg Pathol. 2005; 29(9):1152–1158.

24. Leventon GS, Evans HL. Sarcomatoid squamous cell carcinoma of the mucous membranes of the head and neck: a clinicopathologic study of 20 cases. Cancer. 1981; 48(4):994–1003.


25. Sreenan JJ, Hart WR. Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am J Surg Pathol. 1995; 19(6):666–674.

26. Tan KB, Murali R, Karim RZ, Dutta B, Dutta R, McCarthy SW, et al. Merkel cell carcinoma with fibrosarcomatous differentiation. Pathology. 2008; 40(3):314–316.


27. Mc Menamin ME, Goh SG, Poblet E, Gostelow BE, Robson A, Calonje E. Sarcomatoid basal cell carcinoma--predilection for osteosarcomatous differentiation: a series of 11 cases. Am J Surg Pathol. 2006; 30(10):1299–1308.

28. Kantrow SM, Boyd AS. Primary cutaneous metaplastic carcinoma: report of a case involving angiosarcoma. Am J Dermatopathol. 2007; 29(3):270–273.


29. Agostini T, Mori A, Leporatti G, Dini M, Franchi A. Cutaneous carcinosarcoma: report of a case with myofibroblastic sarcomatous component. Dermatol Surg. 2008; 34(3):418–422.


30. Inaloz HS, Ayyalaraju RS, Holt PJ, Laidler P. A case of sarcomatoid carcinoma of the skin. J Eur Acad Dermatol Venereol. 2003; 17(1):59–61.


31. Boamah H, Ballard B. A case report of spindle cell (sarcomatoid) carcinoma of the larynx. Case Rep Med. 2012; 2012:370204.

32. De Stefani A, Boffano P, Bongioannini G. Review of histologic and immunohistochemical features of spindle cell carcinomas (carcinosarcomas) of the larynx. J Craniofac Surg. 2014; 25(5):e430–e433.

33. Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007; 29(4):374–377.


35. Kobos JW, Yu GH, Varadarajan S, Brooks JS. Primary cutaneous osteosarcoma. Am J Dermatopathol. 1995; 17(1):53–57.


36. Tschen JA, Goldberg LH, McGavran MH. Carcinosarcoma of the skin. J Cutan Pathol. 1988; 15(1):31–35.











 PDF
PDF Citation
Citation Print
Print



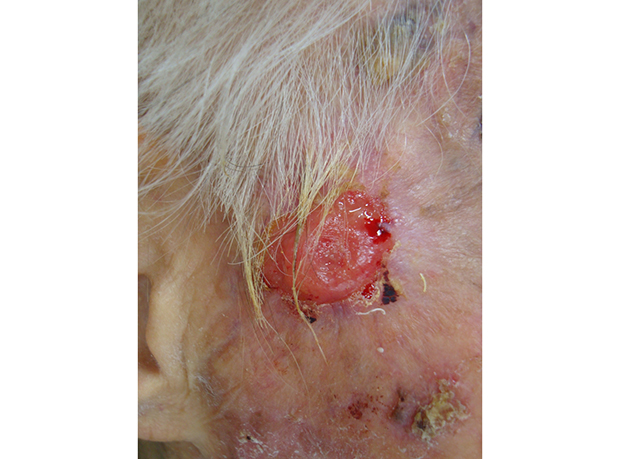
 XML Download
XML Download