Abstract
Primary neuroendocrine tumors originating from the extrahepatic bile duct are rare. Among these tumors, large cell neuroendocrine carcinomas (NECs) are extremely rare. A 59-year-old man was admitted to Sanggye Paik Hospital with jaundice that started 10 days previously. He had a history of laparoscopic cholecystectomy, which he had undergone 12 years previously due to chronic calculous cholecystitis. Laboratory data showed abnormally elevated levels of total bilirubin 15.3 mg/dL (normal 0.2–1.2 mg/dL), AST 200 IU (normal 0–40 IU), ALT 390 IU (normal 0–40 IU), and gamma-glutamyl transferase 1,288 U/L (normal 0–60 U/L). Serum CEA was normal, but CA 19-9 was elevated 5,863 U/mL (normal 0–37 U/mL). Abdominal CT revealed a 4.5 cm sized mass involving the common bile duct and liver hilum and dilatation of both intrahepatic ducts. Percutaneous transhepatic drainage in the left hepatic duct was performed for preoperative biliary drainage. The patient underwent radical common bile duct and Roux-en-Y hepaticojejunostomy for histopathological diagnosis and surgical excision. On histopathological examination, the tumor exhibited large cell NEC (mitotic index >20/10 high-power field, Ki-67 index >20%, CD56 [+], synaptophysin [+], chromogranin [+]). Adjuvant concurrent chemotherapy and radiotherapy were started because the tumor had invaded the proximal resection margin. No recurrence was detected at 10 months by follow-up CT.
Neuroendocrine neoplasms (NENs) arising in the extrahepatic bile duct are rare,1 and among these neoplasms, large cell neuroendocrine carcinomas (NECs) are extremely rare.1 It is difficult to diagnose NECs prior to surgery because there are no specific serum markers or symptoms.2 Treatment and prognosis of NECs have not been established. Here, we report a 58-year-old man with jaundice and a tumor in the extrahepatic bile duct histologically diagnosed as NEC.
A 58-year-old man visited Sanggye Paik Hospital complaining of postprandial dyspepsia and jaundice of several duration. The patient had discomfort but neither tenderness nor a palpable mass in the right upper abdominal quadrant. He had a history of chronic alcoholism and of laparoscopic cholecystectomy due to chronic calculous cholecystitis 12 years previously. Three years prior to presentation he had been diagnosed with fatty liver, and a year previously had undergone a regular sonogram, but there were no unusual findings other than fatty liver. The patient had no family history of cancer. At admission, his laboratory test results were as follows: hemoglobin 15.1 g/dL, white blood cells 7,200/mm3, platelets 192,000/mm3, AST 200 U/L, ALT 390 U/L, LDH 222 U/L, GGT 1,288 U/L, total bilirubin 15.3 mg/dL, direct bilirubin 9.1 mg/dL, CEA 2.01 ng/mL, CA 19-9 5,863 U/mL, and AFP 1.7 ng/mL. Chest X-ray detected no active lung lesion and electrocardiography demonstrated normal sinus rhythm. Initially, the patient underwent abdominal CT, which revealed the lobulated contour of an 4.5 cm sized soft tissue mass in the common bile duct and dilatation of both intrahepatic bile ducts (Fig. 1). MRCP revealed a bile duct neoplasm originating in the cystic duct and invading the common bile duct (Fig. 2). Subsequently, a percutaneous transhepatic biliary drain was inserted in the left hepatic duct, and the patient was referred for surgical excision under a presumptive diagnosis of extrahepatic bile duct cancer. At surgery, we detected a hard mass on the common bile duct invading the right hepatic artery and the portal vein. Radical common bile duct and Roux-en-Y hepaticojejunostomy were conducted. No tumor was detected by frozen biopsy of 8 lymph nodes or at the distal common bile duct margin. However, the tumor had involved proximal and radical resection margins and invaded beyond the bile duct wall. The frozen specimen submitted for macroscopic pathologic analysis was a reddish, soft tissue mass measuring 62×4×26 mm. The common bile duct was patent and mucosa was found intact but focally elevated due to a submucosal mass. Grossly, the surface of the mass had a homogeneous, grayish yellow, solid appearance (Fig. 3), and on microscopic examination was found to be composed of neoplastic cells with frequent mitotic features (mitotic index >20/10 high-power field [HPF], Ki-67 index >20%) (Fig. 4). Immunohistochemistry showed tumor cells were positive for CD56, synaptophysin, and chromogranin. Histopathological examination resulted in a diagnosis of large cell neuroendocrine tumor (NETs) (6.2 cm). The operative procedure and postoperative course were uneventful. The patient was discharged on day 17 after the operation. Because of tumor invasion in the proximal resection margin, he received radiotherapy 28 days after the operation, consisting of six cycles of etoposide and cisplatin. At his 10-month follow-up, the patient was asymptomatic and abdominopelvic computed tomography provided no evidence recurrence. In addition, CA 19-9 decreased gradually after surgery, from 56.1 U/mL at 1 month postoperatively to 37.6 U/mL at 3 months, and to 22.1 U/mL at 1 year.
NENs are epithelial neoplasms that exhibit predominantly neuroendocrine differentiation. The majority of these neoplasms are located in the gastrointestinal tract (55% of all NENs), but they can arise in any organ.1 However, primary NENs of the extrahepatic ducts are extremely rare and account for only 0.32% of primary sites.2 In a study published in 2014, the common hepatic duct and the distal common bile duct were reported to be the most frequent sites of extrahepatic biliary NENs.3 However, a recent Korean study revealed that the ampulla of Vater is the most common site of biliary NENs.4 In the World Health Organization 2017 classification, NENs are primarily classified as well-differentiated NETs, poorly differentiated NECs, and mixed neuroendocrine-non-NENs based on pathological findings. Furthermore, well-differentiated NETs are subclassified into NET G1 (Ki-67 index <3% and mitotic index <2/10 HPFs), NET G2 (Ki-67 index 3–20% or mitotic index 2–20/10 HPFs) and NET G3 (Ki-67 index >20% or mitotic index >20/10 HPFs). Poorly differentiated NECs are subclassified as NEC G3 (Ki-67 index >20% or mitotic index >20/10 HPFs).5 Large cell NEC (LCNEC) and small cell NEC are differentiated by cell size. According to the existing literature, our case is only the fourth reported case of pure LCNEC arising in the extrahepatic bile duct.6 The etiology of NENs in the extrahepatic bile duct is unclear, though it might be argued they originate from neuroendocrine cells at sites of intestinal or gastric-type metaplasia induced by chronic inflammation.789 Indeed, intestinal or gastric-type metaplasias are not uncommon in non-neoplastic mucosa adjacent to a variety of NENs of the gallbladder, including carcinoid tumor, small cell NEC, and LCNEC.71011 Even though the incidence of NENs has increased over time, presumably due to greater awareness and improved endoscopic tools, the preoperative diagnosis of NENs in the extrahepatic bile duct is extremely difficult because symptoms and serum markers are non-specific. Furthermore, no treatment for LCNEC has yet been established. The majority of reported cases of LCNEC of the biliary tract have exhibited aggressive courses and short survival times.12 In our case, the patient received surgery plus adjuvant concurrent chemoradiotherapy and showed no sign of tumor recurrence at 10 months postoperatively. We recommend that because NECs are more aggressive than other NETs, cases of NEC subtypes should be collected with a view toward establishing treatment and enabling prognosis.
Figures and Tables
Fig. 1
Abdominal computed tomograph showing the lobulated contour of the soft tissue mass, which involved liver hilum and the common hepatic duct (4.5 cm), and dilatation of both intrahepatic ducts. (A) Non-enhanced phase. (B) Arterial phase (blue arrows). (C) Portal venous phase.
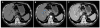
Fig. 2
Magnetic resonance and MRCP images showing the lobulated contour of the mass at the cholecystectomy site (4.4 cm), which exhibited low T1WI and high T2WI signal intensities, direct invasion of the common hepatic duct, and dilation of both intrahepatic ducts. (A) Sagittal image (blue arrow). (B) Coronal reconstruction image (blue arrow). (C) MRCP image (blue arrow). MRCP, magnetic resonance cholangiopancreatography.
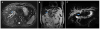
Fig. 3
Gross appearance of the neoplasm. Macroscopically, the frozen specimen was a reddish soft tissue mass measuring 62×4×26 mm. The common bile duct was patent and mucosa was intact though focally elevated due to submucosal mass. The cut surface of the mass had a homogeneous, grayish yellow, solid appearance.

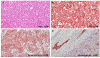
Fig. 4
Histopathologic appearance of the neuroendocrine carcinoma. (A) H&E (original magnification, ×200) showing the tumor under normal mucosa and lymphatic invasion. The subepithelial mass was composed of round neoplastic cells arranged in sheets. Tumor cells had round to oval hyperchromatic nuclei with frequent mitotic features. Immunostaining showed; (B) positivity for CD56 (a membrane protein usually present in neuroendocrine cells; original magnification, ×200), (C) for synaptophysin (typically expressed on the surfaces of neurons or endothelial cells; original magnification, ×200), and (D) positivity for chromogranin (original magnification, ×100).
References
1. Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004; 240:117–122.

2. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003; 97:934–959.

3. Michalopoulos N, Papavramidis TS, Karayannopoulou G, Pliakos I, Papavramidis ST, Kanellos I. Neuroendocrine tumors of extrahepatic biliary tract. Pathol Oncol Res. 2014; 20:765–775.

4. Lee KJ, Cho JH, Lee SH, et al. Clinicopathological characteristics of biliary neuroendocrine neoplasms: a multicenter study. Scand J Gastroenterol. 2017; 52:437–441.

5. Ueda Y, Toyama H, Fukumoto T, Ku Y. Prognosis of patients with neuroendocrine neoplasms of the pancreas according to the World Health Organization 2017 classification. JOP. 2017; S(3):216–220.
6. Murakami M, Katayama K, Kato S, et al. Large-cell neuroendocrine carcinoma of the common bile duct: a case report and a review of literature. Surg Case Rep. 2016; 2:141.

7. Barrón-Rodríguez LP, Manivel JC, Méndez-Sánchez N, Jessurun J. Carcinoid tumor of the common bile duct: evidence for its origin in metaplastic endocrine cells. Am J Gastroenterol. 1991; 86:1073–1076.
8. Eltawil KM, Gustafsson BI, Kidd M, Modlin IM. Neuroendocrine tumors of the gallbladder: an evaluation and reassessment of management strategy. J Clin Gastroenterol. 2010; 44:687–695.
9. Albores-Saavedra J, Nadji M, Henson DE, Ziegels-Weissman J, Mones JM. Intestinal metaplasia of the gallbladder: a morphologic and immunocytochemical study. Hum Pathol. 1986; 17:614–620.

10. Kuwabara H, Uda H. Small cell carcinoma of the gall-bladder with intestinal metaplastic epithelium. Pathol Int. 1998; 48:303–306.

11. Papotti M, Cassoni P, Sapino A, Passarino G, Krueger JE, Albores-Saavedra J. Large cell neuroendocrine carcinoma of the gallbladder: report of two cases. Am J Surg Pathol. 2000; 24:1424–1428.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download