Abstract
Purpose
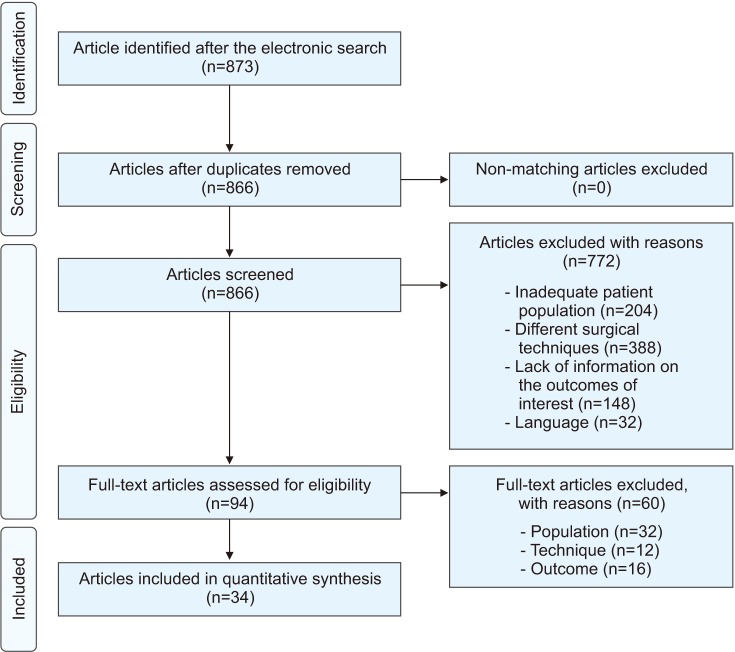
Materials and Methods
Results
Conclusions
Notes
Author Contributions:
Conceptualization: Cito G, Coccia ME, Sessa F, Cocci A.
Data curation: Cito G, Sessa F, Verrienti P.
Formal analysis: Sessa F, Picone R, Fucci R.
Supervision: Serni S, Carini M, Natali A.
Validation: Serni S, Carini M, Natali A, Criscuoli L.
Visualization: Serni S, Carini M, Natali A.
Writing - original draft: Cito G, Sessa F.
Writing - review & editing: Cocci A, Natali A.
References
Table 1
Baseline characteristic of the study cohort
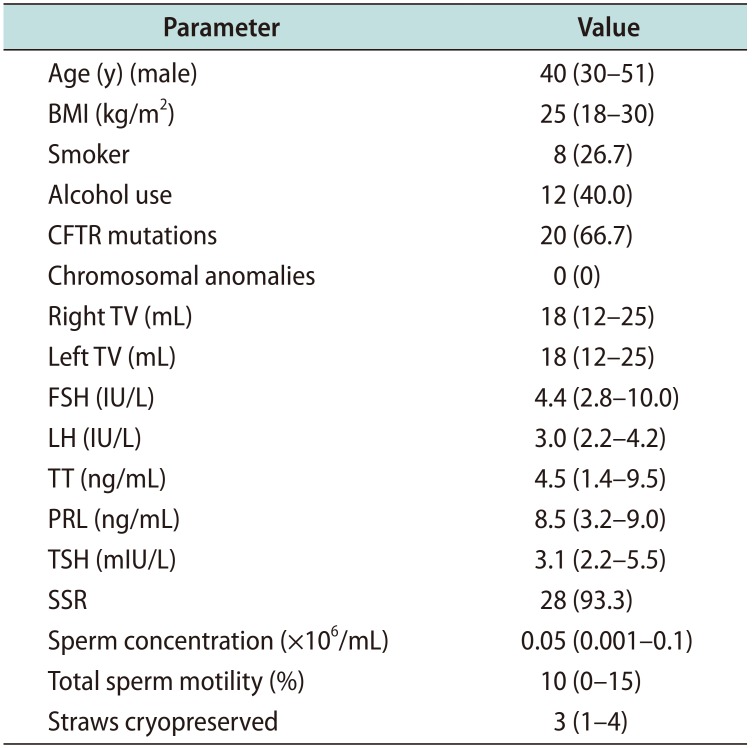
Values are presented as median (interquartile range) or number (%). BMI: body mass index, CFTR: Cystic Fibrosis Transmembrane Conductance Regulator, TV: testicular volume, FSH: follicle stimulating hormone, LH: luteinizing hormone, TT: total testosterone, PRL: prolactin, TSH: thyroid stimulating hormone, SSR: successful sperm retrieval.
Table 2
Characteristics of study population and surgical technique details obtained from included studies (n=34)
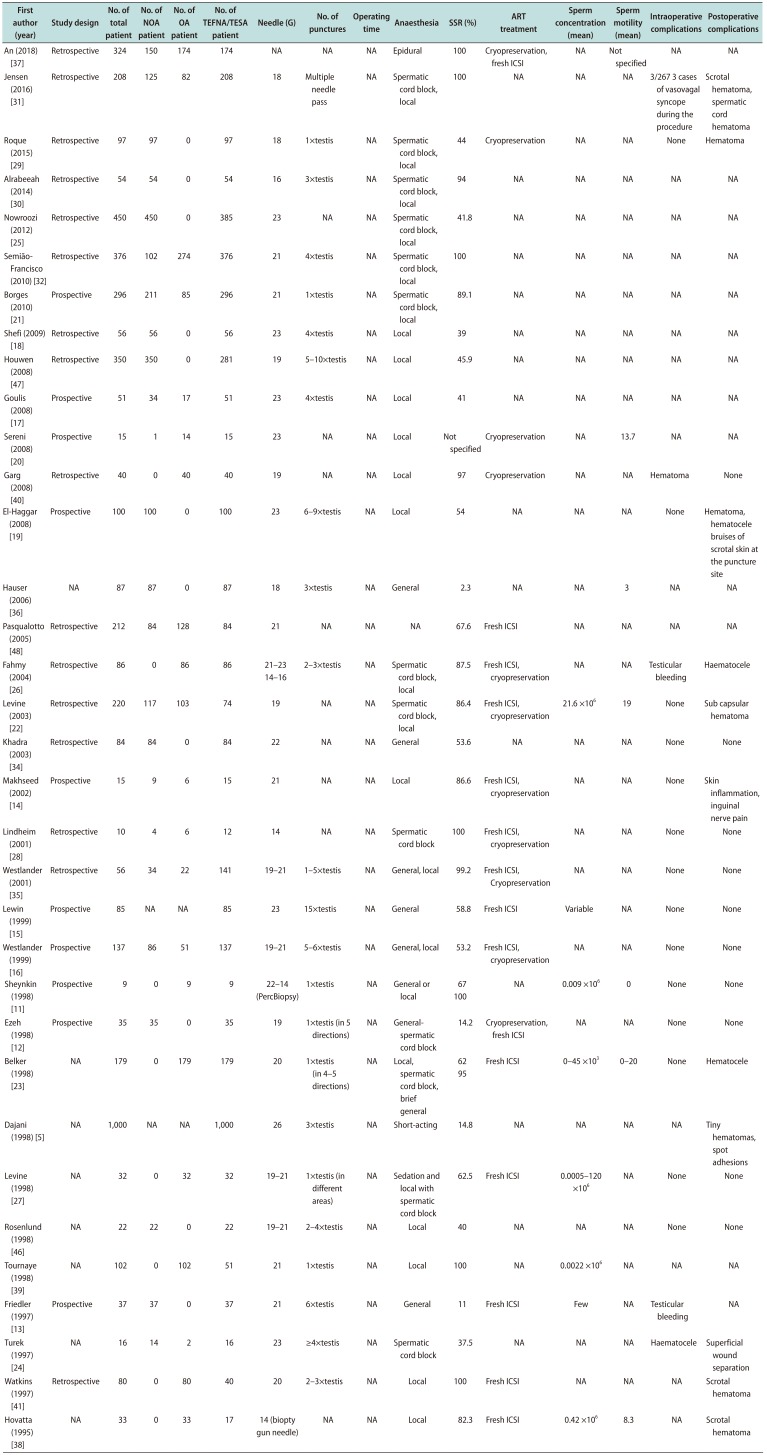
| First author (year) | Study design | No. of total patient | No. of NOA patient | No. of OA patient | No. of TEFNA/TESA patient | Needle (G) | No. of punctures | Operating time | Anaesthesia | SSR (%) | ART treatment | Sperm concentration (mean) | Sperm motility (mean) | Intraoperative complications | Postoperative complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| An (2018) [37] | Retrospective | 324 | 150 | 174 | 174 | NA | NA | NA | Epidural | 100 | Cryopreservation, fresh ICSI | NA | Not specified | NA | NA |
| Jensen (2016) [31] | Retrospective | 208 | 125 | 82 | 208 | 18 | Multiple needle pass | NA | Spermatic cord block, local | 100 | NA | NA | NA | 3/267 3 cases of vasovagal syncope during the procedure | Scrotal hematoma, spermatic cord hematoma |
| Roque (2015) [29] | Retrospective | 97 | 97 | 0 | 97 | 18 | 1×testis | NA | Spermatic cord block, local | 44 | Cryopreservation | NA | NA | None | Hematoma |
| Alrabeeah (2014) [30] | Retrospective | 54 | 54 | 0 | 54 | 16 | 3×testis | NA | Spermatic cord block, local | 94 | NA | NA | NA | NA | NA |
| Nowroozi (2012) [25] | Retrospective | 450 | 450 | 0 | 385 | 23 | NA | NA | Spermatic cord block, local | 41.8 | NA | NA | NA | NA | NA |
| Semião-Francisco (2010) [32] | Retrospective | 376 | 102 | 274 | 376 | 21 | 4×testis | NA | Spermatic cord block, local | 100 | NA | NA | NA | NA | NA |
| Borges (2010) [21] | Prospective | 296 | 211 | 85 | 296 | 21 | 1×testis | NA | Spermatic cord block, local | 89.1 | NA | NA | NA | NA | NA |
| Shefi (2009) [18] | Retrospective | 56 | 56 | 0 | 56 | 23 | 4×testis | NA | Local | 39 | NA | NA | NA | NA | NA |
| Houwen (2008) [47] | Retrospective | 350 | 350 | 0 | 281 | 19 | 5–10×testis | NA | Local | 45.9 | NA | NA | NA | NA | NA |
| Goulis (2008) [17] | Prospective | 51 | 34 | 17 | 51 | 23 | 4×testis | NA | Local | 41 | NA | NA | NA | NA | NA |
| Sereni (2008) [20] | Prospective | 15 | 1 | 14 | 15 | 23 | NA | NA | Local | Not specified | Cryopreservation | NA | 13.7 | NA | NA |
| Garg (2008) [40] | Retrospective | 40 | 0 | 40 | 40 | 19 | NA | NA | Local | 97 | Cryopreservation | NA | NA | Hematoma | None |
| El-Haggar (2008) [19] | Prospective | 100 | 100 | 0 | 100 | 23 | 6–9×testis | NA | Local | 54 | NA | NA | NA | None | Hematoma, hematocele bruises of scrotal skin at the puncture site |
| Hauser (2006) [36] | NA | 87 | 87 | 0 | 87 | 18 | 3×testis | NA | General | 2.3 | NA | NA | 3 | NA | NA |
| Pasqualotto (2005) [48] | Retrospective | 212 | 84 | 128 | 84 | 21 | NA | NA | NA | 67.6 | Fresh ICSI | NA | NA | NA | NA |
| Fahmy (2004) [26] | Retrospective | 86 | 0 | 86 | 86 | 21–23 | 2–3×testis | NA | Spermatic cord block, local | 87.5 | Fresh ICSI, cryopreservation | NA | NA | Testicular bleeding | Haematocele |
| 14–16 | |||||||||||||||
| Levine (2003) [22] | Retrospective | 220 | 117 | 103 | 74 | 19 | NA | NA | Spermatic cord block, local | 86.4 | Fresh ICSI, cryopreservation | 21.6 ×106 | 19 | None | Sub capsular hematoma |
| Khadra (2003) [34] | Retrospective | 84 | 84 | 0 | 84 | 22 | NA | NA | General | 53.6 | NA | NA | NA | None | None |
| Makhseed (2002) [14] | Prospective | 15 | 9 | 6 | 15 | 21 | NA | NA | Local | 86.6 | Fresh ICSI, cryopreservation | NA | NA | None | Skin inflammation, inguinal nerve pain |
| Lindheim (2001) [28] | Retrospective | 10 | 4 | 6 | 12 | 14 | NA | NA | Spermatic cord block | 100 | Fresh ICSI, cryopreservation | NA | NA | None | None |
| Westlander (2001) [35] | Retrospective | 56 | 34 | 22 | 141 | 19–21 | 1–5×testis | NA | General, local | 99.2 | Fresh ICSI, cryopreservation | NA | NA | None | None |
| Lewin (1999) [15] | Prospective | 85 | NA | NA | 85 | 23 | 15×testis | NA | General | 58.8 | Fresh ICSI | Variable | NA | None | None |
| Westlander (1999) [16] | Prospective | 137 | 86 | 51 | 137 | 19–21 | 5–6×testis | NA | General, local | 53.2 | Fresh ICSI, cryopreservation | NA | NA | None | None |
| Sheynkin (1998) [11] | Prospective | 9 | 0 | 9 | 9 | 22–14 (PercBiopsy) | 1×testis | NA | General or local | 67 | NA | 0.009 ×106 | 0 | None | None |
| 100 | |||||||||||||||
| Ezeh (1998) [12] | Prospective | 35 | 35 | 0 | 35 | 19 | 1×testis (in 5 directions) | NA | Generalspermatic cord block | 14.2 | Cryopreservation, fresh ICSI | NA | NA | None | None |
| Belker (1998) [23] | NA | 179 | 0 | 179 | 179 | 20 | 1×testis (in 4–5 directions) | NA | Local, spermatic cord block, brief general | 62 | Fresh ICSI | 0–45×103 | 0–20 | None | Hematocele |
| 95 | |||||||||||||||
| Dajani (1998) [5] | NA | 1,000 | NA | NA | 1,000 | 26 | 3×testis | NA | Short-acting | 14.8 | NA | NA | NA | NA | Tiny hematomas, spot adhesions |
| Levine (1998) [27] | NA | 32 | 0 | 32 | 32 | 19–21 | 1×testis (in different areas) | NA | Sedation and local with spermatic cord block | 62.5 | Fresh ICSI | 0.0005–120×106 | NA | None | None |
| Rosenlund (1998) [46] | NA | 22 | 22 | 0 | 22 | 19–21 | 2–4×testis | NA | Local | 40 | NA | NA | NA | None | None |
| Tournaye (1998) [39] | NA | 102 | 0 | 102 | 51 | 21 | 1×testis | NA | Local | 100 | NA | 0.0022×106 | NA | NA | NA |
| Friedler (1997) [13] | Prospective | 37 | 37 | 0 | 37 | 21 | 6×testis | NA | General | 11 | Fresh ICSI | Few | NA | Testicular bleeding | NA |
| Turek (1997) [24] | NA | 16 | 14 | 2 | 16 | 23 | ≥4×testis | NA | Spermatic cord block | 37.5 | NA | NA | NA | Haematocele | Superficial wound separation |
| Watkins (1997) [41] | Retrospective | 80 | 0 | 80 | 40 | 20 | 2–3×testis | NA | Local | 100 | Fresh ICSI | NA | NA | NA | Scrotal hematoma |
| Hovatta (1995) [38] | NA | 33 | 0 | 33 | 17 | 14 (biopty gun needle) | NA | NA | Local | 82.3 | Fresh ICSI | 0.42 ×106 | 8.3 | NA | Scrotal hematoma |
Table 3
Mean variables of our case series compared to literature




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download