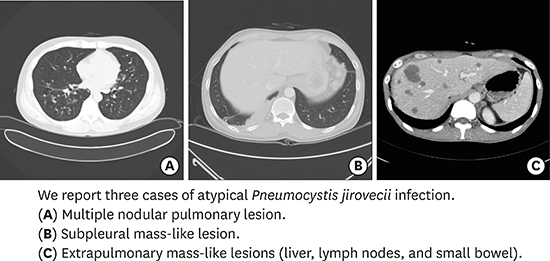
1. The Korean Society for AIDS. Clinical guidelines for the treatment and prevention of opportunistic infections in HIV-infected Koreans. Infect Chemother. 2012; 44(3):93–139.
2. Furrer H, Egger M, Opravil M, Bernasconi E, Hirschel B, Battegay M, et al. Discontinuation of primary prophylaxis against Pneumocystis carinii pneumonia in HIV-1 infected adults treated with combination antiretroviral therapy. Swiss HIV Cohort Study. N Engl J Med. 1999; 340(17):1301–1306.
3. Kim JM, Cho GJ, Hong SK, Chang KH, Chung JS, Choi YH, et al. Epidemiology and clinical features of HIV infection/AIDS in Korea. Yonsei Med J. 2003; 44(3):363–370.

4. Boiselle PM, Crans CA Jr, Kaplan MA. The changing face of Pneumocystis carinii pneumonia in AIDS patients. AJR Am J Roentgenol. 1999; 172(5):1301–1309.
5. Kim HS, Shin KE, Lee JH. Single nodular opacity of granulomatous Pneumocystis jirovecii pneumonia in an asymptomatic lymphoma patient. Korean J Radiol. 2015; 16(2):440–443.
6. Kim EJ, Yoo SJ, Kang GH, Hong MY, Hong JS, Ryu DS, et al. A case of multiple pulmonary nodular Pneumocystis jirovecii pneumonia in an acquired immune deficiency syndrome patient. Infect Chemother. 2012; 44(1):40–43.
7. Kim HW, Heo JY, Lee YM, Kim SJ, Jeong HW. Unmasking granulomatous Pneumocystis jirovecii pneumonia with nodular opacity in an HIV-infected patient after initiation of antiretroviral therapy. Yonsei Med J. 2016; 57(4):1042–1046.
8. Telzak EE, Cote RJ, Gold JW, Campbell SW, Armstrong D. Extrapulmonary Pneumocystis carinii infections. Rev Infect Dis. 1990; 12(3):380–386.
9. Hartel PH, Shilo K, Klassen-Fischer M, Neafie RC, Ozbudak IH, Galvin JR, et al. Granulomatous reaction to Pneumocystis jirovecii: clinicopathologic review of 20 cases. Am J Surg Pathol. 2010; 34(5):730–734.
10. Barrio JL, Suarez M, Rodriguez JL, Saldana MJ, Pitchenik AE.
Pneumocystis carinii pneumonia presenting as cavitating and noncavitating solitary pulmonary nodules in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986; 134(5):1094–1096.
11. Otahbachi M, Nugent K, Buscemi D. Granulomatous Pneumocystis jiroveci pneumonia in a patient with chronic lymphocytic leukemia: A literature review and hypothesis on pathogenesis. Am J Med Sci. 2007; 333(2):131–135.
12. Totet A, Duwat H, Daste G, Berry A, Escamilla R, Nevez G.
Pneumocystis jirovecii genotypes and granulomatous pneumocystosis. Med Mal Infect. 2006; 36(4):229–231.
13. Raviglione MC. Extrapulmonary pneumocystosis: the first 50 cases. Rev Infect Dis. 1990; 12(6):1127–1138.

14. Edelstein H, McCabe RE. Atypical presentations of Pneumocystis carinii pneumonia in patients receiving inhaled pentamidine prophylaxis. Chest. 1990; 98(6):1366–1369.
15. Bondoc AY, White DA. Granulomatous Pneumocystis carinii pneumonia in patients with malignancy. Thorax. 2002; 57(5):435–437.