1. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003; 41:1–12. PMID:
12500213.
2. Ji E, Kim YS. Prevalence of chronic kidney disease defined by using CKD-EPI equation and albumin-to-creatinine ratio in the Korean adult population. Korean J Intern Med. 2016; 31:1120–1130. PMID:
27017386.
3. Kim S, Lim CS, Han DC, Kim GS, Chin HJ, Kim SJ, Cho WY, Kim YH, Kim YS. The prevalence of chronic kidney disease (CKD) and the associated factors to CKD in urban Korea: a population-based cross-sectional epidemiologic study. J Korean Med Sci. 2009; 24(Suppl):S11–S21. PMID:
19194539.
4. Kidney Disease Improving Global Outcome (KDIGO). KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3:1–150.
5. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003; 108:2154–2169. PMID:
14581387.
6. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014; 311:507–520. PMID:
24352797.
7. Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, Banegas JR, Ruilope LM; Spanish ABPM Registry Investigators. Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: a 5,693-patient cross-sectional analysis from Spain. Am J Kidney Dis. 2013; 62:285–294. PMID:
23689071.
8. Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010; 55:441–451. PMID:
19962808.
9. Lee SW, Kim YC, Oh SW, Koo HS, Na KY, Chae DW, Kim S, Chin HJ. Trends in the prevalence of chronic kidney disease, other chronic diseases and health-related behaviors in an adult Korean population: data from the Korean National Health and Nutrition Examination Survey (KNHANES). Nephrol Dial Transplant. 2011; 26:3975–3980. PMID:
21454352.
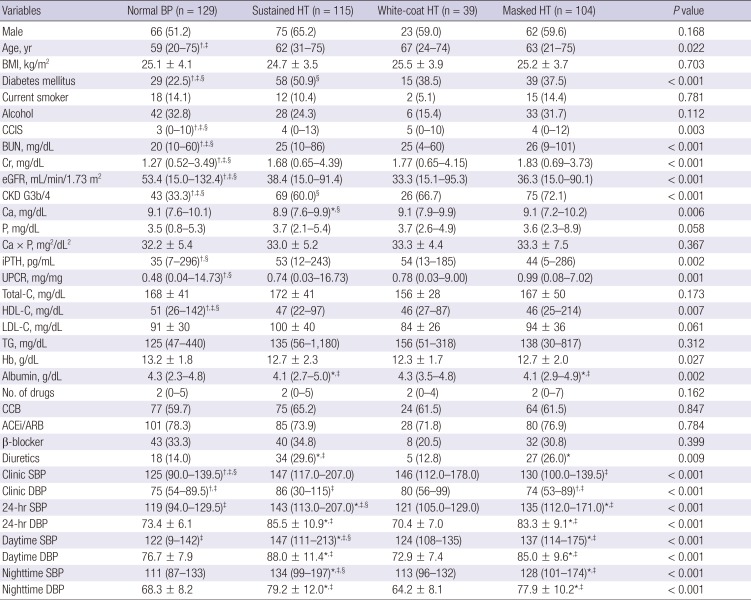
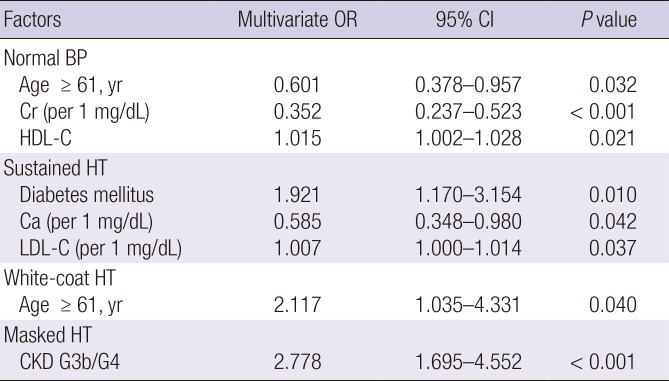
10. Cha RH, Kim S, Yoon SA, Ryu DR, Oh JE, Han SY, Lee EY, Kim DK, Kim YS. Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res. 2014; 37:172–178. PMID:
24048482.
11. Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, Klag MJ, Bakris GL. Hypertension awareness, treatment, and control in chronic kidney disease. Am J Med. 2008; 121:332–340. PMID:
18374693.
12. Pickering TG, James GD, Boddie C, Harshfield GA, Blank S, Laragh JH. How common is white coat hypertension? JAMA. 1988; 259:225–228. PMID:
3336140.
13. Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002; 40:795–796. PMID:
12468559.
14. Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, Faulkner M, Rostand S, Hiremath L, Sika M, Kendrick C, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009; 53:20–27. PMID:
19047584.
15. Wang C, Zhang J, Liu X, Li C, Ye Z, Peng H, Chen Z, Lou T. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013; 8:e55419. PMID:
23393577.
16. Fedecostante M, Spannella F, Cola G, Espinosa E, Dessì-Fulgheri P, Sarzani R. Chronic kidney disease is characterized by “double trouble” higher pulse pressure plus night-time systolic blood pressure and more severe cardiac damage. PLoS One. 2014; 9:e86155. PMID:
24465931.
17. Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2009; 4:656–664. PMID:
19261815.
18. Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Ohashi Y, Hishida A; Chronic Kidney Disease Japan Cohort Study Group. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol. 2013; 8:721–730. PMID:
23411432.
19. Drawz PE, Alper AB, Anderson AH, Brecklin CS, Charleston J, Chen J, Deo R, Fischer MJ, He J, Hsu CY, et al. Masked hypertension and elevated nighttime blood pressure in CKD: Prevalence and association with target organ damage. Clin J Am Soc Nephrol. 2016; 11:642–652. PMID:
26912547.
20. Cohen DL, Huan Y, Townsend RR. Ambulatory blood pressure in chronic kidney disease. Curr Hypertens Rep. 2013; 15:160–166. PMID:
23595357.
21. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612. PMID:
19414839.
22. de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009; 53:466–472. PMID:
19171788.
23. Williams JS, Brown SM, Conlin PR. Videos in clinical medicine. Blood-pressure measurement. N Engl J Med. 2009; 360:e6. PMID:
19179309.
24. Kastner C, Armitage J, Kimble A, Rawal J, Carter PG, Venn S. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis. 2006; 9:270–274. PMID:
16770340.
25. Wu Z, Wu X, Xing F, Zhou S, Luo B, Wang L. Blood pressure characteristics in moderate to severe renal insufficiency. Kidney Blood Press Res. 2015; 40:478–489. PMID:
26418605.
26. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39:S1–S266. PMID:
11904577.
27. Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006; 166:846–852. PMID:
16636209.
28. Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011; 171:1090–1098. PMID:
21709109.
29. An HR, Park S, Yoo TH, Kang SW, Ryu JH, Lee YK, Yu M, Ryu DR, Kim SJ, Kang DH, et al. Non-dipper status and left ventricular hypertrophy as predictors of incident chronic kidney disease. J Korean Med Sci. 2011; 26:1185–1190. PMID:
21935274.
30. Sinha AD, Agarwal R. The complex relationship between CKD and ambulatory blood pressure patterns. Adv Chronic Kidney Dis. 2015; 22:102–107. PMID:
25704346.
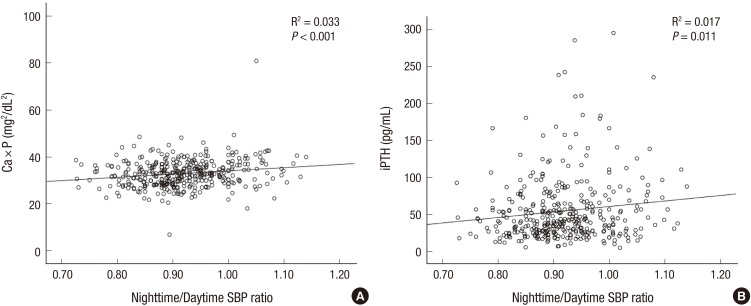
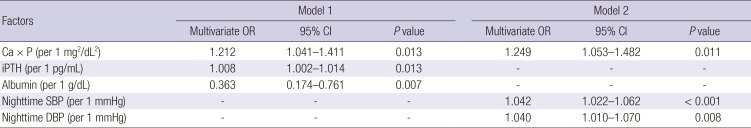
31. Feldstein C, Akopian M, Olivieri AO, Garrido D. Association between nondipper behavior and serum calcium in hypertensive patients with mild-to-moderate chronic renal dysfunction. Clin Exp Hypertens. 2012; 34:417–423. PMID:
22471782.
32. Kanbay M, Isik B, Akcay A, Ozkara A, Karakurt F, Turgut F, Alkan R, Uz E, Bavbek N, Yigitoglu R, et al. Relation between serum calcium, phosphate, parathyroid hormone and ‘nondipper’ circadian blood pressure variability profile in patients with normal renal function. Am J Nephrol. 2007; 27:516–521. PMID:
17703091.
33. Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005; 67:1179–1187. PMID:
15698460.
34. Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT; Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group. The Kidney Disease Outcomes Quality Initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005; 46:925–932. PMID:
16253734.
35. Ott SM. Therapy for patients with CKD and low bone mineral density. Nat Rev Nephrol. 2013; 9:681–692. PMID:
24100401.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download